在Ru(III)固体胶束催化剂上,碱在CO2加氢生成甲酸中的作用
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
二氧化碳催化转化为甲酸酯一般在基本条件下进行。虽然胺的具体作用可能并不总是明确界定,但它们一直是提高活性和转化的首选添加剂。在这项工作中,我们证明了在Ru(III)固体胶束催化剂上CO2加氢生成甲酸的过程中,碱通过改善缓慢的异解H2离解而起到促进剂的作用。动力学实验表明,将CO2形态导向碳酸氢盐是确保甲酸生成的关键,并且加氢速率与H2和TEA的可用性有很强的依赖性。H2-D2同位素置乱结果表明,碱基的存在增加了4倍。不同碱基的筛选证明了TEA独特的促进作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
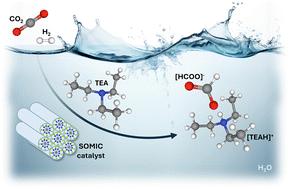
Role of base in CO2 hydrogenation to formate over a Ru(III) solid micellar catalyst
The catalytic conversion of CO2 to formate is generally performed under basic conditions. While the specific role of amines may not always be clearly defined, they have been the preferred additives to enhance activity and conversion. In this work, we demonstrate that during CO2 hydrogenation to formate over a Ru(III) solid micellar catalyst, the base acts as a promoter by improving the sluggish heterolytic H2 dissociation. Kinetic experiments revealed that it is crucial to direct CO2 speciation towards bicarbonate to ensure formate generation, and that there is a strong dependence of the hydrogenation rate on H2 and TEA availability. H2–D2 isotope scrambling showed a fourfold increase in the presence of the base. Screening of different bases evidenced the unique promoting effect of TEA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


