pd基甲烷氧化催化剂失活机理及抑制策略研究进展
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
温室气体对环境的不利影响正在加剧。甲烷是第二大温室气体,具有很高的全球变暖潜力。它作为一种清洁能源被广泛应用于各种领域。然而,它的完全氧化本身是低效的,导致未氧化的甲烷排放,加剧了温室效应。因此,提高甲烷氧化的催化效率至关重要,而氧化催化剂在这一过程中起着举足轻重的作用。贵金属催化剂在甲烷氧化反应中表现出较高的催化效率。其中钯(Pd)是甲烷完全氧化的高活性催化剂,具有优异的活性、选择性和稳定性。然而,甲烷氧化过程中催化剂失活仍然是一个重大挑战。了解催化剂失活的根本原因对于优化催化剂设计、提高性能、延长使用寿命和降低成本至关重要。早期对催化剂失活的研究受到表征技术和理论计算的限制,主要集中在催化剂活性的提高上,研究在静态条件下进行。随着科学技术的发展,对催化剂失活的研究逐渐转向对结构演变和动态反应条件下失活机理的探讨。随着科学技术的进一步发展和人们认识的提高,未来对催化剂失活的研究可能逐渐转向原子水平调控、支撑界面配位等方向的研究。因此,本文综述了甲烷完全氧化过程中钯基催化剂失活的最新进展。全面分析了反应机理、失活原因、影响因素和抑制策略,为今后的研究提供理论见解和实践指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
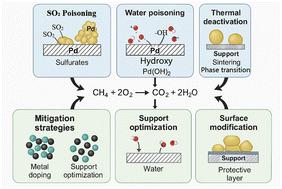
Research progress on the deactivation mechanism and suppression strategies of Pd-based catalysts for methane oxidation
The adverse impact of greenhouse gases on the environment is intensifying. Methane, the second-largest greenhouse gas, possesses high global warming potential. It is widely utilized as a clean energy source in various applications. However, its complete oxidation is inherently inefficient, leading to the emission of unoxidized methane, which exacerbates the greenhouse effect. Therefore, enhancing the catalytic efficiency of methane oxidation is crucial, with oxidation catalysts playing a pivotal role in this process. Noble metal catalysts demonstrate high catalytic efficiency in methane oxidation reactions. Among these, palladium (Pd) is a highly active catalyst for the complete oxidation of methane, exhibiting excellent activity, selectivity, and stability. However, catalyst deactivation during methane oxidation continues to pose a significant challenge. Understanding the fundamental causes of catalyst deactivation is essential for optimizing the catalyst design, enhancing the performance, extending the lifespan, and reducing costs. In the early stage, the study on catalyst inactivation was limited by characterization techniques and theoretical calculations, and the main focus was on the improvement of catalyst activity, and the study was carried out under static conditions. With the development of science and technology, the study of catalyst deactivation has gradually shifted to the discussion of structural evolution and the deactivation mechanism under dynamic reaction conditions. With the further development of science and technology and the improvement of people's understanding, the study on catalyst deactivation may gradually shift to the research of atomic-level regulation, support interface coordination and other directions in the future. Therefore, this review highlights recent advances in Pd-based catalyst deactivation during the complete oxidation of methane. It presents a comprehensive analysis of reaction mechanisms, deactivation causes, influencing factors, and inhibition strategies, providing theoretical insights and practical guidance for future research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


