烯烃和萘与Ar3P-OH自由基氢化的计算见解
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
Ar3P-OH自由基介导的氢化过程是通过质子耦合电子转移(cPCET)途径而不是氢原子转移(HAT)机制进行的。为了使萘衍生物自由基加氢反应的区域选择性合理化,建立了包含LUMO系数和电荷占比的二元线性模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
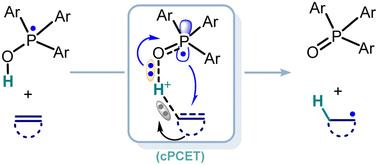
Computational insights into radical hydrogenation of alkenes and naphthalenes with Ar3P–OH radical
The Ar3P–OH radical-mediated hydrogenation proceeds through a concerted proton-coupled electron transfer (cPCET) pathway rather than a hydrogen atom transfer (HAT) mechanism. A binary linear model incorporating LUMO coefficients and charge populations has been developed to rationalize the regioselectivity of radical hydrogenation of naphthalene derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


