Cs+ -甘氨酸相互作用:逐渐水化(n = 0-8)对中性和两性离子形式的影响
IF 3.1
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
Gly·Cs+(H2O)n簇(n = 0 ~ 8)的计算研究揭示了甘氨酸(Gly)与水中Cs+的溶剂依赖相互作用机制。Cs+主要通过双齿配位与Gly的羧酸基结合。前4个水分子连接Cs+和Gly,而第5到7个水分子不再直接与Gly相互作用。关键的是,加入第八个水分子会触发甘氨酸从中性转化为两性离子形式。先进的理论分析(RDG, AIM, ESP)揭示了Cs+-Gly相互作用强度随水化程度的增加呈非单调趋势,特别是在n = 8时导致溶剂分离离子配对。本文章由计算机程序翻译,如有差异,请以英文原文为准。
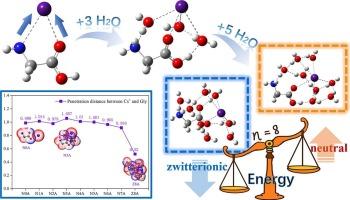
Cs+–Glycine interactions: Effects of progressive hydration (n = 0–8) on neutral and zwitterionic forms
Computational studies of Gly·Cs+(H2O)n clusters (n = 0–8) revealed the solvation-dependent interaction mechanism between glycine (Gly) and Cs+ in water. Cs+ primarily binds Gly via a bidentate coordination to its carboxylate group. The first four water molecules bridge Cs+ and Gly, whereas the fifth to seventh no longer interact directly with Gly. Crucially, adding the eighth water molecule triggers the conversion of glycine from neutral to its zwitterionic form. Advanced theoretical analyses (RDG, AIM, ESP) uncovered a non-monotonic trend in the Cs+-Gly interaction strength with increasing hydration, particularly resulting in solvent-separated ion pairing at n = 8.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Letters
化学-物理:原子、分子和化学物理
CiteScore
5.70
自引率
3.60%
发文量
798
审稿时长
33 days
期刊介绍:
Chemical Physics Letters has an open access mirror journal, Chemical Physics Letters: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Chemical Physics Letters publishes brief reports on molecules, interfaces, condensed phases, nanomaterials and nanostructures, polymers, biomolecular systems, and energy conversion and storage.
Criteria for publication are quality, urgency and impact. Further, experimental results reported in the journal have direct relevance for theory, and theoretical developments or non-routine computations relate directly to experiment. Manuscripts must satisfy these criteria and should not be minor extensions of previous work.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


