Bavachinin通过激活TLR4/STING轴依赖性PANoptosis发挥抗肿瘤作用,协同增强子宫内膜癌的化疗敏感性。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
子宫内膜癌是一种起源于子宫内膜的恶性肿瘤,其发病率和死亡率在全球范围内呈上升趋势。目前,对于复发性、耐药性和转移性这种疾病的患者,没有有效的治疗选择。通过化合物文库筛选,我们发现巴伐克宁(BVC)对EC细胞具有杀伤作用。BVC是一种从中药茯苓中提取的具有潜在药理作用的生物活性小分子,但其具体机制尚不清楚。我们首次发现BVC诱导ZBP1 (Z-DNA结合蛋白1)介导的EC细胞PANoptosis,其特征是激活焦亡、凋亡和坏死。BVC促进活性氧(ROS)的产生、线粒体膜电位(MMP, ΔΨm)崩溃和ATM-CHK2介导的DNA损伤,激活EC细胞的cGAS-STING通路。机制上,网络药理学、分子对接、细胞热移实验(CESTA)和药物亲和反应靶稳定性(DARTS)实验表明,BVC通过直接与toll样受体4 (TLR4)相互作用,从而触发线粒体ROS生成,激活cGAS-STING通路,诱导EC细胞PANoptosis。值得注意的是,TLR4敲低抑制STING-TBK1-IRF3通路和zbp1介导的PANoptosis。此外,低剂量BVC联合顺铂可增加磷酸化的H2AX表达,提示BVC可增强EC细胞对顺铂的敏感性。体内研究表明,BVC可诱导EC PANoptosis,且BVC联合顺铂具有有效的抗肿瘤作用,且不损伤重要器官。这些新发现为支持BVC和panoposis为基础的治疗EC的临床应用提供了强有力的证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
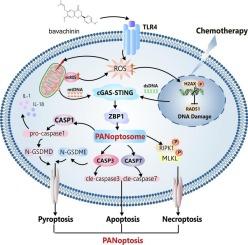
Bavachinin exerts anti-tumor effects by activating TLR4/STING axis-dependent PANoptosis and synergistically enhances chemosensitivity in endometrial cancer
The incidence and mortality rates of endometrial cancer (EC), a malignancy originating from endometrium, have been increasing globally. Currently, there are no effective therapeutic options for patients with recurrent, chemoresistant, and metastatic forms of this disease. Through compound library screening, we identified that bavachinin (BVC) has a killing effect on EC cells. BVC is a bioactive small molecule with potential pharmacological effects derived from the traditional Chinese herb Proralea corylifolia L, but the specific mechanisms are unclear. We first discovered that BVC induces ZBP1 (Z-DNA binding protein 1)-mediated PANoptosis in EC cells, characterized by activating of pyroptosis, apoptosis, and necroptosis. BVC promoted reactive oxygen species (ROS) production, mitochondrial membrane potential (MMP, ΔΨm) collapse, and ATM-CHK2 mediated DNA damage, which activated cGAS-STING pathway in EC cells. Mechanistically, network pharmacology, molecular docking, cellular thermal shift assays (CESTA), and drug affinity responsive target stability (DARTS) experiments revealed that BVC induced PANoptosis in EC cells by directly interacting with toll-like receptor 4 (TLR4), thereby triggering mitochondrial ROS generation, activating the cGAS-STING pathway. Notably, TLR4 knockdown inhibited STING-TBK1-IRF3 pathway and ZBP1-mediated PANoptosis. In addition, low-dose BVC combined with cisplatin increased phosphorylated H2AX expression, suggesting that BVC enhances the sensitivity of EC cells to cisplatin. In vivo studies demonstrated that BVC induced PANoptosis in EC, and BVC in combination with cisplatin had effective anti-tumor effect without injuring vital organs. These novel findings provide compelling evidence to support the clinical application of BVC and PANoptosis-based therapy for treating EC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


