靶向Hsp70和FoxM1的苯并咪唑和苯并噻唑衍生物的设计、合成及抗癌评价
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
以已知Hsp70 (VER-155008)和FoxM1 (FDI-6)抑制剂为支架,设计了一系列新的苯并咪唑和苯并噻唑衍生物。分子对接研究表明,与这两个靶标具有良好的结合亲和力,其中一些化合物在Hsp70的atp酶结构域和FoxM1的dna结合区域内表现出强烈的相互作用。通过对接分数鉴定出最有希望的候选物质,并通过FT-IR、1H NMR和13C NMR进行了合成和结构确认。使用MTT试验评估它们对MCF-7、HeLa和HUVEC细胞系的细胞毒性。苯并噻唑衍生物比苯并咪唑衍生物具有更强的细胞毒活性。其中,化合物7d表现出最有效的抗增殖作用,其IC₅₀值为10.83 μM (MCF-7), 12.68 μM (HeLa)和106.75 μM (HUVEC)。分子动力学模拟进一步证实了7d在FoxM1结合口袋内的稳定性,支持其作为潜在FoxM1抑制剂的作用。虽然在基于细胞的实验中双靶点抑制的实验证实尚待验证,但计算结果表明7d可能作为Hsp70和FoxM1的双重调节剂,需要进一步的机制研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
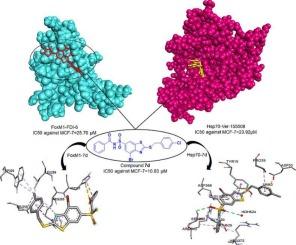
Design, synthesis, and anticancer evaluation of benzimidazole and benzothiazole derivatives targeting Hsp70 and FoxM1
A series of novel benzimidazole and benzothiazole derivatives was designed based on the scaffolds of known Hsp70 (VER-155008) and FoxM1 (FDI-6) inhibitors. Molecular docking studies indicated favorable binding affinities to both targets, with several compounds showing strong interactions within the ATPase domain of Hsp70 and the DNA-binding region of FoxM1. The most promising candidates, as identified by docking scores, were synthesized and structurally confirmed using FT-IR, 1H NMR, and 13C NMR spectroscopy. Their cytotoxicity was evaluated against MCF-7, HeLa, and HUVEC cell lines using the MTT assay. Benzothiazole derivatives exhibited greater cytotoxic activity than benzimidazole counterparts. Among them, compound 7d demonstrated the most potent antiproliferative effect, with IC₅₀ values of 10.83 μM (MCF-7), 12.68 μM (HeLa), and 106.75 μM (HUVEC). Molecular dynamics simulations further confirmed the stability of 7d within the FoxM1 binding pocket, supporting its role as a potential FoxM1 inhibitor. While experimental confirmation of dual-target inhibition in cell-based assays is pending, the computational findings suggest that 7d may function as a dual modulator of Hsp70 and FoxM1, warranting further mechanistic investigation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


