整合转录组和单细胞RNA测序分析揭示了GBP4在胰腺腺癌中的预后意义
IF 5
2区 医学
Q2 Medicine
引用次数: 0
摘要
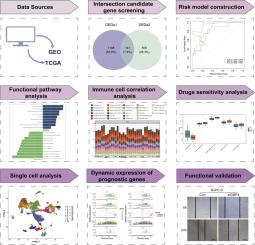
GBP4在癌症中的作用已被初步确定,但其在胰腺腺癌(PAAD)患者中的具体功能尚不清楚。本研究的目的是确定GBP4基因对PAAD的影响。方法从公共数据库中获取转录组学和单细胞RNA测序(scRNA-seq)数据。使用单变量Cox和最小绝对收缩和选择算子(LASSO)回归筛选预后基因,以构建和验证模型。途径富集和免疫微环境分析探讨了PAAD的机制,而scRNA-seq揭示了关键细胞群和动态基因表达。体外和体内研究了GBP4对肿瘤细胞生长的功能实验。结果本研究确定了5个与GBP4基因相关的PAAD预后基因,包括GBP2、KRT6A、MMP7、BCAT1和SPRR1A。风险模型具有有效性和普遍性,“细胞周期”途径在高危组富集,代谢途径在低危组富集。免疫细胞浸润(如中枢记忆CD8 T细胞、活化B细胞)在危险组间差异显著(p < 0.01),并与预后基因相关。导管细胞是关键细胞,其预后基因表达在分化过程中发生变化。体外功能实验证实了GBP4在促进胰腺癌细胞增殖、迁移和侵袭中的作用。此外,在体内,GBP4的沉默抑制了肿瘤的生长,而GBP4的过表达则促进了肿瘤的生长。结论本研究鉴定了GBP4相关预后基因,揭示了GBP4在胰腺癌进展中的作用,为胰腺癌预后预测和靶向治疗提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Integrated transcriptome and single-cell RNA sequencing analysis revealed the prognostic significance of GBP4 in pancreatic adenocarcinoma
Background
The role of GBP4 in cancer has been preliminarily identified, yet its specific function in patients with pancreatic adenocarcinoma (PAAD) remains unclear. The aim of this study is to determine the impact of the GBP4 gene on PAAD.
Methods
Transcriptomics and single-cell RNA sequencing (scRNA-seq) data were obtained from public databases. Prognostic genes were screened using univariate Cox and least absolute shrinkage and selection operator (LASSO) regression to construct and validate the model. Pathway enrichment and immune microenvironment analyses explored PAAD mechanisms, while scRNA-seq revealed key cell populations and dynamic gene expression. Functional experiments of GBP4 on tumor cell growth were investigated in vitro and in vivo.
Results
This study identified 5 prognostic genes related to the GBP4 gene in PAAD, including GBP2, KRT6A, MMP7, BCAT1, and SPRR1A. The risk model showed validity and generalizability, with "cell cycle" pathway enrichment in high-risk groups and metabolic pathways in low-risk groups. Immune cell infiltration (e.g., central memory CD8 T cells, activated B cells) differed significantly between risk groups (p < 0.01) and correlated with prognostic genes. Ductal cells were key cells, with prognostic gene expression varying during differentiation. In vitro functional assays confirmed the role of GBP4 in promoting pancreatic cancer cell proliferation, migration, and invasion. Moreover, silencing of GBP4 inhibited tumor growth in vivo, whereas GBP4 overexpression increased the tumor growth.
Conclusion
This study identifies GBP4-related prognostic genes and demonstrates the role of GBP4 in pancreatic cancer progression, providing new perspectives for prognostic prediction and therapeutic targeting.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


