患者来源的高级别浆液性卵巢癌脱细胞组织细胞外基质作为类器官生长的生物相容性支持
IF 5
2区 医学
Q2 Medicine
引用次数: 0
摘要
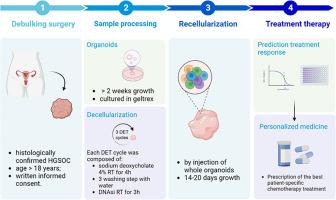
高级别浆液性卵巢癌(HGSOC)是临床上最常见的卵巢癌,通常被认为是一种致命的疾病。虽然目前的治疗似乎提供几乎完全缓解,复发率仍然很高。在这里,我们提出了一种创新的组织工程方法,将患者来源的脱细胞细胞外基质(dECM)和患者来源的类器官(PDO)结合起来,应用于HGSOC,旨在提供一个有用的三维(3D)模型来评估治疗反应。通过组织学、免疫组织化学、免疫荧光和二次谐波显微镜,我们证明了dECM维持了原生肿瘤组织的结构环境。与天然肿瘤相比,对分离的dECM进行的蛋白质组学分析显示,与ECM组装、组织和形态相关的主要功能相关蛋白组与组织特异性生态位的保存一致,以供后期PDO的播种和浸润。同时,我们建立了PDO的衍生方案,起始效率为83.3%。我们使用CK7和P16的一致性指数为100%,P53mut、PAX8和WT1的一致性指数为66%的诊断标记将PDO与其天然肿瘤对应物进行比较。紫杉醇和紫杉醇加卡铂治疗后PDO的IC50浓度分别为37.10µM和6.8µM。注射PDO使dECM再细胞化,并用药物治疗,对标准一线化疗的敏感性降低。这种3D模型可以成为一个可靠的临床前患者特异性平台,以弥合体外和体内药物测试分析之间的差距。新颖性和影响声明:本研究的新颖性在于,首次建立了完全来源于患者的卵巢癌三维临床前模型。患者的脱细胞细胞外基质能够支持类器官的生长,并且与仅在商业基质中生长的类器官相比,对化疗治疗产生的反应更接近于体内使用的实际剂量。我们希望所提出的模型能够在确定正确的药物治疗患者,避免不必要的毒性方面产生有益的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Patient-derived extracellular matrix from decellularized high-grade serous ovarian carcinoma tissues as a biocompatible support for organoid growth
High-grade serous ovarian cancer (HGSOC) is the most common clinically diagnosed ovarian cancer, often considered a fatal disease. Although current treatments appear to provide almost complete remission, the recurrence rate is still high. Here we present an innovative tissue engineering approach applied to HGSOC by combining patient-derived decellularized extracellular matrix (dECM) and patient-derived organoids (PDO), intending to provide a three-dimensional (3D) model useful to evaluate treatment response. By histology, immunohistochemistry, immunofluorescence, and second harmonic generation microscopy, we demonstrated that dECM maintains the structural environment of native tumoral tissue. Proteomic analysis performed on isolated dECM compared to the native tumor revealed a dominant set of functionally related proteins associated with ECM assembly, organization and morphology consistent with preservation of a tissue-specific niche for later PDO seeding and infiltration. In parallel, we established a protocol for the PDO derivation with an initiation efficiency of 83.3 %. We compared PDO with its native tumor counterpart using diagnostics markers with a concordance index of 100 % for CK7 and P16 and 66 % for P53mut, PAX8 and WT1. The IC50 concentrations of PDO treated with Paclitaxel and Paclitaxel plus Carboplatin resulted in 37.10 µM and 6.8 µM, respectively, after treatment. The dECM recellularized by injection with PDO, and treated with drugs displayed a reduced sensitivity to standard first-line chemotherapy. This 3D model could be a reliable preclinical patient-specific platform to bridge the gap between in vitro and in vivo drug testing assays.
Novelty and Impact statement
The novelty of this research lies in the fact that, for the first time, a three-dimensional preclinical model of ovarian cancer fully derived from the patient has been generated. The patient's decellularized extracellular matrix is capable of supporting the growth of organoids and produces a response to chemotherapy treatment that more closely resembles the actual doses used in vivo compared to organoids grown solely in commercial matrices. We hope that the presented model can have a useful impact in identifying the right drugs to treat patients, and avoiding unnecessary toxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


