新型Rh-NHC配合物的合成和表征:苯硼酸在醛上的加成,DFT/TD-DFT和QTAIM研究
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
本文通过苯并咪唑盐与[Rh(OMe)(COD)]2化合物反应,合成了三个新的Rh(I)-NHC配合物(2a-c)。通过1H、13C NMR、FT-IR、UV-vis等光谱方法和元素分析技术对合成化合物的结构进行了表征。对新合成的Rh-NHC配合物的电子结构、反应性、施-受体相互作用和热力学性质进行了深入的理论研究。为此,利用密度泛函理论(DFT)计算。结果表明,3,4,5-三甲氧基苄基取代有助于在Rh配合物中形成更稳定和刚性的结构。此外,还进行了TD-DFT计算,以评估配合物2a-c在氯仿环境中的潜在电子跃迁。可以将这三个配合物的峰解释为主要与配体内π→π*跃迁和金属-配体电荷转移(MLCT)跃迁有关。采用分子中原子量子理论(QTAIM)来理解2a-c配合物的键特性和分子间相互作用强度。并在苯硼酸与醛的反应中对配合物进行了评价,得到了满意的产率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
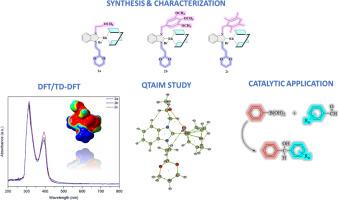
Synthesis and characterization of novel Rh-NHC complexes: addition of phenylboronic acid to aldehydes, DFT/TD-DFT, and QTAIM studies
Herein, three new Rh(I)-NHC complexes (2a-c) were synthesized by reacting benzimidazolium salts with the [Rh(OMe)(COD)]2 compound. The structures of the synthesized compounds were elucidated by various spectroscopic methods such as 1H, 13C NMR, FT-IR, and UV–vis spectroscopies, and the elemental analysis technique. A thorough theoretical investigation was conducted to explore the electronic structure, reactivity, donor-acceptor interactions, and thermodynamic properties of newly synthesized Rh-NHC complexes. To this end, Density Functional Theory (DFT) calculations were utilized. The findings reveal that the 3,4,5-trimethoxybenzyl substitution contributes to the formation of a more stable and rigid structure in the Rh complex. Additionally, TD-DFT calculations were performed to assess the potential electronic transitions of the complexes 2a-c within a chloroform environment. It is possible to interpret the observed peaks of the three complexes as predominantly associated with intra-ligand π → π* transitions and metal-ligand charge-transfer (MLCT) transitions. The quantum theory of atoms in molecules (QTAIM) was employed to facilitate comprehension of bond characteristics and intermolecular interaction strengths in 2a-c complexes. Furthermore, the complexes were evaluated in the reaction of phenylboronic acid to aldehydes, and satisfactory yields were obtained for the desired products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


