用于绿色铃木-宫浦交叉偶联反应的新型茶碱功能化磁性钯配合物
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
本文通过多步合成工艺,合成了一种新型高效的茶碱基n -杂环碳-钯金属配合物,并将其固定在纳米磁铁矿上。利用FT-IR、XRD、HR-TEM、SEM、EDX、TGA、VSM和XPS等分析技术对该配合物进行了全面表征。评价了其催化芳基卤化物与芳基硼酸之间的Suzuki-Miyaura交叉偶联反应的效能。催化剂的亲水表面,由于存在磺酸基,显著提高了其在水中的活性,提供了一个环保的反应介质。反应在常温下在水介质中进行,产率高,反应时间短。该配合物很容易使用外部磁铁隔离,并具有出色的可重复使用性,最多可重复使用6次,而产量没有令人钦佩的变化。此外,催化系统显示出高周转率(TON)和周转率(TOF),确保了其可持续性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
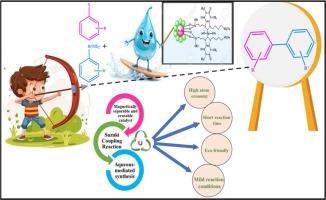
A novel theophylline-functionalized magnetic palladium complex for green suzuki-miyaura cross-coupling reaction
In this work, a novel highly efficient theophylline-based N-heterocyclic carbene-Pd(II) metal complex is synthesized and immobilized on the nano-magnetite through a multistep synthetic process. The complex is thoroughly characterized using various analytical techniques, such as FT-IR, XRD, HR-TEM, SEM, EDX, TGA, VSM, and XPS. Its catalytic potency was evaluated for the Suzuki-Miyaura cross-coupling reaction between aryl halides and aryl boronic acids. The catalyst's hydrophilic surface, due to the presence of sulphonate groups, significantly enhances its activity in water, providing an eco-friendly reaction medium. The reaction proceeds at ambient temperature in aqueous media, resulting high yield and shorter reaction time. The complex is easily isolated using an external magnet and exhibits excellent reusability for up to a total of six cycles without an admirable change in yield. Furthermore, the catalytic system shows high turnover numbers (TON) and turnover frequencies (TOF), ensuring its sustainability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


