靶向青霉素结合蛋白(PBP2a)和mecA基因的吡唑和吡唑嘧啶衍生物抗mrsa的研究进展
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
全球卫生受到耐甲氧西林金黄色葡萄球菌(MRSA)的威胁。MRSA流行率的上升使抗感染治疗策略复杂化,突出了迫切需要针对MRSA的新型治疗方法。本研究合成了新型吡唑和吡唑嘧啶衍生物,并利用IR、1H/13C NMR和元素分析对其进行了综合表征。这些衍生物对革兰氏阳性菌(金黄色葡萄球菌ATCC 25923和MRSA ATCC 43300)和革兰氏阴性菌(大肠杆菌ATCC 25922和肺炎克雷伯菌ATCC 700603)均有显著的抑菌活性(使用MIC测试),此外对白色念珠菌ATCC 10231也有抑菌活性。值得注意的是,与阳性对照药物(硫酸新霉素和氟康唑)相比,希夫碱吡唑6b和6c是最有希望的衍生物。此外,MBC/MFC试验显示出除吡唑衍生物3a外的杀菌和杀真菌活性,该衍生物对MRSA具有特异性抑菌作用。有希望的化合物6b和6c对MRSA表现出很强的抗生物膜活性,在½MIC下,生物膜的形成分别减少了74.1%和71.36%。希夫碱吡唑(6b和6c)通过Western blotting显示PBP2a蛋白表达水平降低,显示出对MRSA的特异性活性。此外,聚合酶链反应和mecA基因测序证实了暴露于这些化合物后的诱导突变,表明在表型和基因型水平上存在双重作用机制。最后,对这些有前景的衍生物进行的计算机ADME研究成功地预测了它们的口服生物利用度、药物相似性和药代动力学特征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
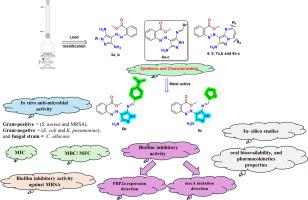
Development of pyrazole and pyrazolopyrimidine derivatives as promising anti-MRSA agents targeting penicillin-binding protein (PBP2a) and mecA gene
Global health is threatened by methicillin-resistant S. aureus (MRSA). The rising prevalence of MRSA complicates anti-infective treatment strategies, highlighting the urgent need for novel therapeutics targeting MRSA. In this study, novel pyrazole and pyrazolopyrimidine derivatives were synthesized and comprehensively characterized using IR, 1H/13C NMR, and elemental analysis. Significant anti-microbial activity of these derivatives was demonstrated (using MIC test) against both Gram-positive (S. aureus ATCC 25923 and MRSA ATCC 43300) and Gram-negative bacteria (E. coli ATCC 25922 and K. pneumoniae ATCC 700603), in addition to exhibiting antifungal activity against C. albicans ATCC 10231. Notably, the Schiff bases pyrazoles 6b and 6c represent the most promising derivatives in comparison to the positive control drugs (neomycin sulfate and fluconazole). Additionally, MBC/MFC tests exhibited bactericidal and fungicidal activity, except for pyrazole derivative 3a, which demonstrated bacteriostatic efficacy specifically against MRSA. The promising compounds 6b and 6c showed strong antibiofilm activity against MRSA, resulting in a reduction of biofilm formation by 74.1 % and 71.36 %, respectively, at ½ MIC. Schiff base pyrazoles (6b and 6c) showed specific activity against MRSA, by revealing a reduction in expression of PBP2a protein levels using Western blotting. Additionally, polymerase chain reaction and sequencing of the mecA gene confirmed induced mutations following exposure to these compounds, suggesting a dual mechanism of action at both phenotypic and genotypic levels. Finally, the in-silico ADME studies for the promising derivatives were successful in predicting their oral bioavailability, drug-likeness, and pharmacokinetic features.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


