双季铵BODIPY衍生物:光物理、抗菌和抗肿瘤的光动力应用
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
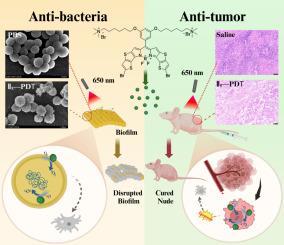
虽然硼-二吡咯甲烷(BODIPY)衍生物已经分别被用于光动力抗菌或抗肿瘤应用,但双活性阳离子BODIPY光敏剂却很少受到关注。此外,传统季铵盐类化合物主要作为抗菌药物进行研究,关于其直接抗肿瘤活性的系统报道尚少。我们开发了双季铵盐阳离子BODIPY衍生物作为光敏剂来研究双光动力抗菌/抗肿瘤作用。所有合成的新化合物在646 ~ 659 nm范围内具有显著的紫外-可见吸收,荧光发射强度(665、670和675 nm)强,并具有活性氧(ROS)生成能力。其中,溴化BODIPYs II3和II5在体外和体内均表现出较高的ROS生成效率和显著的光动力抗肿瘤活性(IC50分别为0.22 μM和0.36 μM),与对照药m-THPC相当。化合物I5和II5对致病性金黄色葡萄球菌(S. aureus)具有较强的光动力抑菌活性,最低抑菌浓度(MIC)均为0.5 μg/mL。此外,它们通过破坏膜的完整性获得了有效的生物膜抑制效率,分别为86.74%和85.23%。这些发现对开拓新的多功能光敏剂具有重要的前景,这些光敏剂可以实现联合或顺序抗菌和抗肿瘤光动力治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bis-quaternary ammonium BODIPY derivatives: Photophysical, antimicrobial, and antitumor photodynamic applications
Although Boron-dipyrromethene (BODIPY) derivatives have been explored for photodynamic antibacterial or antitumor applications, respectively, dual-activity cationic BODIPY photosensitizers have received much less attention. In addition, traditional quaternary ammonium compounds (QACs) are primarily studied as antibacterial agents, with systematic reports on their direct antitumor activity remaining scarce. We developed bis-quaternary ammonium cationic BODIPY derivatives as photosensitizers to investigate dual photodynamic antibacterial/antitumor action. All synthesized new compounds demonstrated notable UV-vis absorption in the range of 646-659 nm, intense fluorescence emission (665, 670, and 675 nm), and capabilities for reactive oxygen species (ROS) generation. Among these, brominated BODIPYs II3 and II5 exhibited high ROS generation efficiency and significant photodynamic antitumor activity against Eca-109 cells in vitro (IC50 = 0.22 μM and 0.36 μM) and in vivo, comparable to the control drug m-THPC. Compounds I5 and II5 demonstrated substantial photodynamic antibacterial activities against pathogenic Staphylococcus aureus (S. aureus), with minimum inhibitory concentrations (MIC) of 0.5 μg/mL for both compounds. Furthermore, they achieved effective biofilm inhibition efficiencies of 86.74% and 85.23%, respectively, through the disruption of membrane integrity. These findings hold significant promise for pioneering new multifunctional photosensitizers that enable combined or sequential antimicrobial and antitumor photodynamic therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


