异黄酮非芳香环A类似物7,8-二氢- 4h -铬-4,5(6H)-二酮的简易制备及其抗增殖作用的评价
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
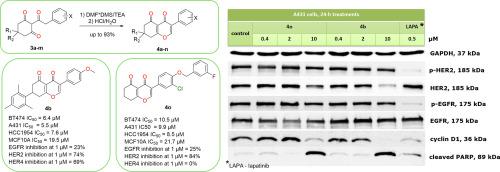
在本文中,我们描述了一个简单的方法来合成一类新的化合物- 3-芳基-7,8-二氢- 4h -铬-4,5(6H)-二酮。在三乙胺的存在下,采用低成本的二甲基甲酰胺-硫酸二甲酯加合物,由易于获得的2-(2-芳基乙酰基)环己烷-1,3-二酮制备了目标化合物。所获得的化合物可以被认为是异黄酮的非芳香环A类似物,在微摩尔IC50范围内具有中等的抗增殖活性,对her2阳性癌细胞具有适度的选择性。一项分子模拟研究表明,它们可能的作用机制可能与抑制HER2有关。此外,通过在所报道的化合物的结构中引入3-氯-4-((3-氟苯基)氧)苯基,我们设计并合成了化合物40作为潜在的选择性HER2抑制剂。两种化合物,4b和40,对HER2具有不同的预测结合模式,体外测试了对8种酪氨酸激酶的激酶活性抑制。在浓度为1 μM时,化合物4b对HER2和HER4的抑制率分别为74%和69%。在相同浓度下,化合物40对HER2的抑制率仅为84%。然而,用4b或40处理的A431细胞的免疫印迹出人意料地显示HER2总表达减少,而不是磷酸化HER2 (p-HER2)。此外,这两种化合物都能降低细胞周期蛋白D1水平,刺激PARP裂解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A facile method for the preparation of 7,8-dihydro-4H-chromene-4,5(6H)-diones as non-aromatic cycle A analogs of isoflavones and their evaluation as antiproliferative agents
In this article, we describe a facile method for the synthesis of a novel class of compounds – 3-aryl-7,8-dihydro-4H-chromene-4,5(6H)-diones. The target compounds were prepared from easily accessible 2-(2-arylacetyl)cyclohexane-1,3-diones using a low-cost dimethylformamide-dimethyl sulfate adduct in the presence of triethylamine. The obtained compounds, which can be considered non-aromatic cycle A analogs of isoflavones, demonstrated moderate antiproliferative activity in the micromolar IC50 range and modest selectivity toward HER2-positive cancer cells. A molecular modeling study suggested that their possible mechanism of action may involve HER2 inhibition. Additionally, by introducing a 3-chloro-4-((3-fluorobenzyl)oxy)phenyl group into the structure of the reported compounds, we designed and synthesized compound 4o as a potential selective HER2 inhibitor. Two compounds, 4b and 4o, which had different predicted binding modes to HER2, were tested in vitro for kinase activity inhibition against eight tyrosine kinases. At a concentration of 1 μM, compound 4b inhibited HER2 and HER4 by 74 % and 69 %, respectively. At the same concentration, compound 4o significantly inhibited only HER2 by 84 %. However, immunoblotting in A431 cells treated with 4b or 4o unexpectedly showed a reduction in total HER2 expression rather than in phosphorylated HER2 (p-HER2). Furthermore, both compounds reduced cyclin D1 level and stimulated PARP cleavage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


