LOXL2减轻创伤后膝关节骨关节炎和疼痛
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
软骨具有有限的自我再生潜力,损伤会导致结构、分子和功能失常,从而导致骨关节炎(OA)。外伤性膝关节损伤也可导致软骨变性和外伤性膝关节炎。本研究旨在探讨赖氨酸氧化酶样2 (LOXL2)缺失是否会加重PTOA,而过表达是否会在机械和分子水平上减轻炎症和疼痛。方法采用改良的C57BL/6J小鼠膝关节内侧半月板切除术,并应用聚集蛋白促进软骨中Loxl2的特异性缺失。通过RNA-seq和qPCR研究转录组畸变,通过跑步机疲劳和von Frey伤害感受试验评估腺病毒传递的LOXL2关节内治疗后的生物力学和异常性疼痛。结果发现sloxl2在小鼠膝关节pta中表达下调。膝关节软骨Loxl2缺失,表现为oa样分子改变,并加重pta。转录组学分析显示软骨变性因子上调,炎症M1巨噬细胞的特征和疼痛。这些Loxl2缺失的PTOA小鼠具有与人类膝关节OA致病基因标记的分子相似性。有趣的是,LOXL2治疗可以缓解膝关节功能,减少M1巨噬细胞浸润,恢复生物力学能力,并通过减轻膝关节残疾和疼痛来减轻机械异常性疼痛。结论LOXL2缺失增强了pta的严重程度,类似于人类OA,而过表达通过减轻炎症和疼痛来减轻这些影响,使LOXL2成为OA的治疗选择。eloxl2可调节炎症、疼痛和退行性变,显示出作为人类PTOA疾病改善疗法的强大翻译潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
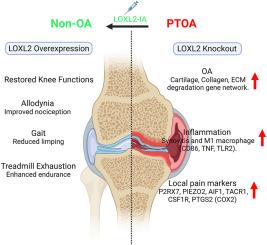
LOXL2 alleviates post-traumatic knee osteoarthritis and pain
Background
Cartilage has limited potential for self-regeneration, and damage results in structural, molecular, and functional aberrations, leading to osteoarthritis (OA). Traumatic knee injuries can also lead to cartilage degeneration and post-traumatic OA (PTOA). This study aimed to explore whether lysyl oxidase-like 2 (LOXL2) deletion aggravate PTOA and overexpression alleviate inflammation and pain at mechanical as well as molecular levels.
Methods
Modified medial meniscectomy was performed on C57BL/6J mice knee followed by aggrecan promotes specific deletion of Loxl2 in cartilage. Transcriptomic aberrations were studied using RNA-seq and qPCR, and biomechanics and allodynia was evaluated using treadmill exhaustion and von Frey nociception test after adenovirus-delivered LOXL2 intra-articular treatment.
Results
LOXL2 was found to be downregulated in mouse knee PTOA. Loxl2 deletion in knee cartilage, shows OA-like molecular changes, and aggravates PTOA. Transcriptomics analysis revealed the upregulation of cartilage degeneration factors, signatures of inflammatory M1 macrophages, and pain. These Loxl2 deleted PTOA mice have a molecular resemblance to the human knee OA pathogenic gene signature. Interestingly, LOXL2 treatment alleviates knee joint function, reduces M1 macrophage infiltration, restores biomechanic capabilities, and reduces mechanical allodynia by relieving knee joint disability and pain.
Conclusion
LOXL2 deletion enhances the severity of PTOA, similar to human OA, whereas overexpression mitigates these effects by reducing inflammation and pain, offering LOXL2 as a therapeutic option in OA.
The translational potential of this article
LOXL2 modulates inflammation, pain, and degeneration, showing strong translational potential as a disease-modifying therapy for human PTOA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


