多尺度仿生融合构建促进骨质疏松性骨缺损修复的铈离子水凝胶支架
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
骨质疏松症的骨缺损治疗面临许多挑战。在骨质疏松微环境中,骨吸收大于骨形成,阻碍了骨缺损区域的自我修复。此外,缺损部位成骨-血管生成耦合功能的恶化和过度的炎症反应进一步使骨缺损的治疗复杂化。因此,迫切需要一种改进的方法来加强骨质疏松性骨缺损的治疗。方法将3d打印的磷酸三钙(TCP)支架、甲基丙烯酸胶原(COMA)水凝胶和阿仑膦酸钠铈离子纳米颗粒整合在一起,构建了一种多尺度仿生融合阿仑膦酸钠铈离子水凝胶支架。在体外,我们用tcp - h -阿仑膦酸钠纳米颗粒(ACNP)支架提取物干预骨质疏松大鼠骨髓基质细胞(BMSCs),通过碱性磷酸酶(ALP)酶活性染色、茜素红染色、Western Blot、RT-qPCR和免疫荧光染色检测成骨相关指标,评价TCP-H-ACNP支架的成骨分化效果。通过转录组测序,我们探讨了TCP-H-ACNP支架影响骨质疏松性骨髓间质干细胞成骨分化的机制。用TCP-H-ACNP支架提取物干预人脐静脉内皮细胞(HUVECs),通过成管实验和细胞划痕实验评价TCP-H-ACNP支架的血管生成作用。在体内,我们建立骨质疏松大鼠股骨远端骨缺损模型,通过Mirco CT、苏木精伊红(H&;E)染色、Masson染色和免疫组化染色评价体内治疗效果。结果体外实验表明,TCP-H-ACNP支架可促进骨质疏松大鼠骨髓间充质干细胞成骨分化和huvec血管生成。在体内,TCP-H-ACNP支架可促进骨质疏松大鼠股骨远端骨缺损的骨再生和修复,促进局部血管生成。机制上,TCP-H-ACNP支架可通过Wnt信号通路直接促进骨质疏松大鼠骨髓间充质干细胞成骨分化,并通过影响钙离子转运和改善线粒体功能间接促进成骨分化。结论制备的水凝胶支架不仅具有足够的机械支持,而且具有良好的细胞生长微环境,并含有促进成骨和血管分化的生物因子。这一应用代表了多尺度仿生水凝胶支架在治疗骨质疏松性骨缺损方面的一个开创性的方面,为骨质疏松性骨缺损的治疗提供了一个新的方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
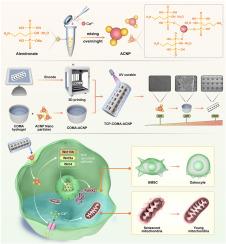
Multi-scale biomimetic fusion construction of cerium ion hydrogel-scaffold for promoting osteoporotic bone defect repair
Background
The treatment of bone defects in the context of osteoporosis encounters numerous challenges. In the osteoporotic microenvironment, bone resorption outweighs bone formation, impeding the self-repair of bone defect areas. Furthermore, the deterioration of osteogenesis-angiogenesis coupling function at the defect sites and excessive inflammatory responses further complicate the treatment of bone defects. Hence, an improved approach is urgently needed to enhance the treatment of osteoporotic bone defects.
Methods
Our efficient strategy has developed a multi-scale biomimetic fusion alendronate sodium cerium ion hydrogel scaffold, integrating 3D-printed tricalcium phosphate (TCP) scaffolds, collagen-methacrylate (COMA) hydrogel, and nanoparticles of alendronate sodium cerium ions. In vitro, we intervened osteoporosis rat derived bone marrow stromal cells (BMSCs) with the extract of TCP-H-Alendronate sodium cerium ion nanoparticles (ACNP) scaffold and detected the osteogenesis-related indicators through alkaline phosphatase (ALP) enzymatic activity staining, alizarin red staining, Western Blot, RT-qPCR and immunofluorescence staining to evaluate the osteogenic differentiation effect of TCP-H-ACNP scaffold. Through transcriptome sequencing, we explored the mechanism of TCP-H-ACNP scaffold affecting osteogenic differentiation of osteoporotic BMSCs. We intervened human umbilical vein endothelial cells (HUVECs) with the extract of TCP-H-ACNP scaffold and evaluated the angiogenic effect of TCP-H-ACNP scaffold through tube formation assay and cell scratch assay. In vivo, we established a distal femoral bone defect model in osteoporotic rats and evaluated the therapeutic effect in vivo through Mirco CT, Hematoxylin and Eosin (H&E) stainin, Masson staining and immunohistochemical staining.
Results
The results demonstrated that in vitro, TCP-H-ACNP scaffolds could promote osteogenic differentiation of osteoporotic BMSCs from rats and angiogenesis of HUVECs. In vivo, TCP-H-ACNP scaffolds could promote bone regeneration and repair of distal femoral bone defects in osteoporotic rats and improve local angiogenesis. Mechanistically, TCP-H-ACNP scaffolds could directly promote osteogenic differentiation of osteoporotic BMSCs from rats through the Wnt signaling pathway, and indirectly promote osteogenic differentiation by influencing Ca ion transport and improving mitochondrial function.
Conclusion
We create a hydrogel scaffold that not only offers adequate mechanical support but also possesses a favorable microenvironment for cell growth and contains biological factors promoting osteogenic and angiogenic differentiation.
The translational potential of this paper
This application represents a pioneering aspect of multi-scale biomimetic hydrogel scaffolds in addressing osteoporotic bone defects, providing a novel direction for the treatment of osteoporotic bone defects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


