胆汁酸-姜黄素缀合物作为乳腺癌潜在抗增殖药物的设计、合成和分子模型研究
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本研究合成了新的胆汁酸-姜黄素缀合物(缀合物3和4),并利用FT-IR、1H NMR和13C NMR对其进行了结构表征。这些偶联物对两种三阴性乳腺癌细胞系(TNBC) 4T1和MDA-MB-231的细胞毒性潜力进行了评估。共轭物3显示出异常的细胞毒活性,4 T1的IC₅₀值为3.3±0.06 μM, MDA-MB-231的IC₅₀值为0.7±0.01 μM,显著超过姜黄素的活性(IC₅₀分别= 29.7±0.9 μM和10.04 μM)。共轭物4也显示出增强的功效,IC₅0值为5.3±0.5 μM (4 T1)和2.5±0.03 μM (MDA-MB-231)。细胞周期分析显示,偶联物3的G0/G1期细胞数量从未处理细胞的89.90%大幅减少至75.10%,与姜黄素的20.39%和偶联物4的75.90%相比,偶联物3诱导的细胞凋亡率为37.10%,表明细胞周期进程被破坏,抗增殖作用增强。偶联物3和4均表现出良好的生物相容性,对正常巨噬细胞无毒性。这些发现证实,胆汁酸结合显著增强姜黄素的抗增殖潜能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
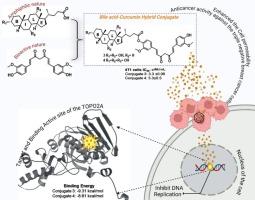
Design, synthesis and molecular modelling studies of bile acid-curcumin conjugates as potential antiproliferative agents for breast cancer
In this study, novel bile acid–curcumin conjugates (conjugates 3 and 4) were synthesized and structurally characterized using FT-IR, 1H NMR, and 13C NMR spectroscopy. The cytotoxic potential of these conjugates was evaluated against two triple negative breast cancer cell lines (TNBC), 4T1, and MDA-MB-231. Conjugate 3 displayed exceptional cytotoxic activity, with IC₅₀ values of 3.3 ± 0.06 μM for 4 T1 and 0.7 ± 0.01 μM for MDA-MB-231, significantly surpassing the activity of curcumin (IC₅₀ = 29.7 ± 0.9 μM and10.04 μM, respectively). Conjugate 4 also demonstrated enhanced efficacy, with IC₅₀ values of 5.3 ± 0.5 μM (4 T1) and 2.5 ± 0.03 μM (MDA-MB-231). Cell cycle analysis revealed a substantial reduction in the G0/G1 phase population from 89.90 % in untreated cells to 75.10 % for conjugate 3, which induced apoptosis of 37.10 % than curcumin 20.39 % and 75.90 % for conjugate 4, indicating disruption of cell cycle progression and enhanced antiproliferative effects. Both Conjugates 3 and 4 demonstrated excellent biocompatibility, exhibiting no toxicity toward normal macrophages. These findings confirm that bile acid conjugation significantly enhances the antiproliferative potential of curcumin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


