揭示人血红蛋白中酪氨酸和色氨酸在代表蛋白质微环境的结构模板发育中的功能重要性
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
围绕酪氨酸(Tyr) /色氨酸(Trp)和血红素的微环境似乎表征了人血红蛋白(hbb)的紫外-可见吸收光谱。利用多种工具对hbb进行结构解析,这可能有助于其光谱特性,然后表明亚基A具有更高的结构稳定性及其血红素Tyr42和Trp14的重要性。A亚基Tyr42和Trp14对甘氨酸(Gly)的突变进一步验证了它们在确定hbb的结构稳定性、理化性质、功能性质和二级结构方面的贡献。据此,我们首次以Tyr42和血红素的结构坐标为第一簇,Trp14、Tyr42和血红素为第二簇来表征hb的微环境。这两种簇的计算(DFT)吸收和FTIR特性与整个hbb的实验吸收和FTIR特性很好地一致,这表明这些簇具有潜在的生物医学应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
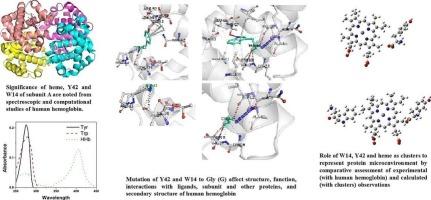
Revealing the functional importance of tyrosine and tryptophan of human hemoglobin for development of structural templates representing protein microenvironment
Microenvironment surrounding Tyrosine (Tyr) / Tryptophan (Trp) and heme appear to characterize the UV–vis absorption spectra of human hemoglobin (HHb). Structural elucidation of HHb using multiple tools, that may contribute to its spectral properties, then indicate greater structural stability of subunit A and the significance of its heme, Tyr42 and Trp14. Mutagenesis of Tyr42 and Trp14 of subunit A to Glycine (Gly) further validate their contribution in determining the structural stability, physicochemical properties, functional properties, and secondary structure of HHb. Accordingly, the use of structural coordinates of Tyr42 and heme as the first cluster and Trp14, Tyr42 and heme as the second cluster to represent the microenvironment of HHb is assessed for the first time. The calculated (DFT) absorption and FTIR properties of both the clusters are in well agreement with experimental absorption and FTIR characteristics of whole HHb suggesting prospective biomedical applications of these clusters.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


