CUDC-907通过诱导有丝分裂突变和下调YAP/TAZ信号通路对非小细胞肺癌具有抑制作用。
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
非小细胞肺癌(NSCLC)是肺癌最常见的组织学亚型,死亡率相对较高。CUDC-907是一种组蛋白去乙酰化酶(HDAC)和磷脂酰肌醇3-激酶(PI3K)的双靶点抑制剂,具有抑制多种恶性肿瘤进展的潜力。然而,CUDC-907对NSCLC的抗癌作用尚未完全阐明。在这项研究中,我们探讨了CUDC-907的抗nsclc作用及其可能的潜在机制。用不同浓度的CUDC-907处理非小细胞肺癌细胞,采用细胞计数试剂盒-8 (CCK-8)检测细胞活力。采用集落形成和5-乙基-2′-脱氧尿苷(EdU)测定法评估细胞增殖;采用X变异组蛋白(H2AX)免疫荧光法监测DNA损伤。采用异种移植小鼠模型评估CUDC-907对NSCLC的体内抗肿瘤效果,并通过Western blot分析检测蛋白表达水平。结果显示,CUDC-907以浓度依赖性的方式降低A549和H1299 细胞的活力。集落形成和EdU实验表明,CUDC-907抑制NSCLC细胞的增殖。暴露于CUDC-907导致A549和H1299 细胞G2/M期阻滞,其原因是细胞周期蛋白A、细胞分裂周期25C (Cdc25C)、p-Cdc25C、Cdc2和细胞周期蛋白B1的表达降低,p21蛋白水平升高。CUDC-907通过下调Aurora A、Aurora B和polo样激酶1 (PLK1)的表达,诱导NSCLC细胞中H2AX灶形成和有丝分裂异常。此外,CUDC-907通过增加caspase-3、caspase-8、caspase-9和聚(adp核糖)聚合酶(PARP)的裂解,触发A549和H1299 细胞凋亡。机制研究表明,CUDC-907在A549和H1299 细胞中激活了肌醇要求酶1α (IRE1α)-c-Jun n末端激酶(JNK)/p38丝裂原活化蛋白激酶(MAPK)通路,阻断了带pdz结合基序(TAZ)信号通路的细胞相关蛋白(YAP)/转录共激活因子。此外,在肿瘤异种移植小鼠模型中,CUDC-907治疗显著抑制肿瘤生长并降低肿瘤重量。综上所述,本研究揭示了CUDC-907的细胞毒性作用及其潜在机制,提示CUDC-907可能是治疗非小细胞肺癌的有效方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
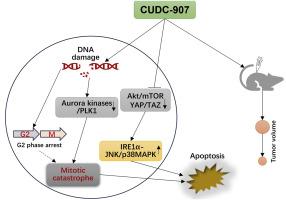
CUDC-907 exerts an inhibitory effect on non-small cell lung cancer associated with induction of mitotic catastrophe and downregulation of YAP/TAZ signaling
Non-small cell lung cancer (NSCLC) is the most common histologic subtype of lung cancer associated with a relatively high mortality rate. CUDC-907, a dual-target inhibitor of histone deacetylase (HDAC) and phosphatidylinositol 3-kinase (PI3K), has the potential to suppress the progression of various malignancies. However, the anti-cancer effect of CUDC-907 on NSCLC remains to be fully elucidated. In this study, we explored the anti-NSCLC effects of CUDC-907 and the possible underlying mechanisms. NSCLC cells were treated with different concentrations of CUDC-907, and cell viability was detected using the Cell Counting Kit-8 (CCK-8) assay. Cell proliferation was evaluated using colony formation and 5-ethynyl-2′-deoxyuridine (EdU) assays, while γ- H2A.X histone variant (H2AX) immunofluorescence was used to monitor DNA damage. The in vivo anti-tumor efficacy of CUDC-907 against NSCLC was evaluated using a xenograft mouse model, and protein expression levels were examined via Western blot analysis. The results revealed that CUDC-907 reduced the viability of A549 and H1299 cells in a concentration-dependent manner. Colony formation and EdU assays showed that CUDC-907 suppressed the proliferation of NSCLC cells. Exposure to CUDC-907 caused G2/M phase arrest in both A549 and H1299 cells by decreasing the expression of cyclin A, cell division cycle 25C (Cdc25C), p-Cdc25C, Cdc2, and cyclin B1, and increasing the protein levels of p21. Treatment with CUDC-907 induced γ-H2AX foci formation and abnormal mitosis in NSCLC cells by downregulating the expression of Aurora A, Aurora B, and polo-like kinase 1 (PLK1). In addition, CUDC-907 triggered A549 and H1299 cell apoptosis by increasing the cleavage of caspase-3, caspase-8, caspase-9, and poly (ADP-ribose) polymerase (PARP). Mechanistic studies revealed that CUDC-907 activated inositol-requiring enzyme 1 α (IRE1α)-c-Jun N-terminal kinase (JNK)/p38 mitogen-activated protein kinase (MAPK) pathway, and blocked -Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) signaling in A549 and H1299 cells. Additionally, CUDC-907 treatment significantly inhibited tumor growth and reduced tumor weight in the tumor xenograft mouse model. Taken together, this study revealed the cytotoxic effects of CUDC-907 and its underlying mechanism, which suggests that CUDC-907 may be an effective therapeutic approach for treating NSCLC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


