氧化铁纳米颗粒通过过氧化氢依赖性DNA损伤选择性地增强抗坏血酸药物在非小细胞肺癌中的毒性。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
药理抗坏血酸(静脉给药,至血浆水平≈15-20 mM)已被证明对癌症具有选择性毒性,并通过增加过氧化氢(H2O2)的生成和增加细胞内氧化还原活性铁(Fe2+)诱导非小细胞肺癌(NSCLC)的放化疗致敏。目前的研究表明,经fda批准的氧化铁纳米颗粒阿鲁莫xytol (FMX)预处理24小时,可增强P-AscH-对人NSCLC细胞(H1299T和A549)的毒性,但对原代人细支气管上皮细胞(HBEpC)没有毒性。在过量表达多西环素诱导过氧化氢酶的H1299TCat15细胞中,FMX + P-AscH-也诱导细胞杀伤和卡铂诱导的放射化学致敏,这些被多西环素抑制,表明H2O2对生物效应的依赖性。P-AscH- + FMX诱导H1299TCat15细胞内氧化还原活性Fe2+的增加,部分被多西环素诱导的过氧化氢酶过表达抑制,表明P-AscH-和H2O2都参与了FMX细胞内氧化还原活性Fe2+的释放。最后,P-AscH- + FMX处理的H1299TCat15细胞显示单双链DNA损伤增加,这在hbepc中未见,并且被强力霉素诱导的过氧化氢酶表达抑制。该研究首次证明,FMX联合P-AscH选择性地使NSCLC细胞(相对于正常细胞)对抗坏血酸毒性和化学放射致敏,通过增强h2o2依赖性DNA损伤,伴随着细胞内Fe2+释放的增加。这些结果支持了FMX可以选择性地增强非小细胞肺癌对P-AscH-的治疗反应的假设。本文章由计算机程序翻译,如有差异,请以英文原文为准。
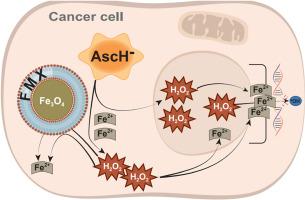
Iron-oxide nanoparticles selectively enhance the toxicity of pharmacological ascorbate through hydrogen peroxide-dependent DNA damage in non-small cell lung cancer (NSCLC)
Pharmacological ascorbate (IV delivery, to plasma levels ≈ 15–20 mM) has been shown to be selectively toxic to cancer vs. normal cells as well as inducing radio-chemo-sensitization in non-small cell lung cancer (NSCLC) via increased generation of hydrogen peroxide (H2O2) and increased intracellular redox-active iron (Fe2+). The current study shows that 24 h pretreatment with an FDA-approved iron-oxide nanoparticle, Ferumoxytol (FMX), enhances the toxicity of P-AscH- in human NSCLC cells (H1299T and A549), but not in primary human bronchiolar epithelial cells (HBEpC). In H1299TCat15 cells engineered to overexpress doxycycline inducible catalase, FMX + P-AscH- also induced cell killing and carboplatin-induced radio-chemo-sensitization that was inhibited by exposure to doxycycline, demonstrating the dependence of the biological effects on H2O2. P-AscH- + FMX induced increases in intracellular redox active Fe2+ in H1299TCat15 cells, that was partially inhibited by doxycycline-inducible catalase overexpression, demonstrating that both P-AscH- and H2O2 participate in the intracellular release of redox active Fe2+ from FMX. Finally, H1299TCat15 cells treated with P-AscH- + FMX demonstrated increased single- and double-strand DNA damage, that was not seen in HBEpCs and was inhibited by doxycycline induced expression of catalase. This study represents the first demonstration that FMX combined with P-AscH- selectively sensitize NSCLC cells (relative to normal cells) to ascorbate toxicity and chemo-radio-sensitization through enhancing H2O2-dependent DNA damage, that is accompanied by increased release of intracellular Fe2+. These results support the hypothesis that FMX can be used to selectively enhance therapy responses to P-AscH- in NSCLC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


