REG3A通过GPR54/ARRB2/ERK1/2配体定向信号通路促进胰腺癌吉西他滨耐药。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
吉西他滨是一种广泛应用于胰腺癌(PCa)的一线化疗药物。然而,吉西他滨耐药性的迅速出现构成了一个主要的临床障碍。在这里,我们发现REG3A是前列腺癌预后不良的关键决定因素。值得注意的是,REG3A在临床吉西他滨耐药PCa患者的肿瘤组织中表达上调。通过全面的体外细胞分析和Reg3g (REG3A的小鼠同源物)敲除小鼠的体内研究,我们证明REG3A缺乏使PCa对吉西他滨敏感。在机制上,RNA测序(RNA- seq)结合蛋白-蛋白相互作用(PPI)分析显示REG3A作为胞外作用蛋白结合到新的膜受体GPR54。这种相互作用增加了GPR54的膜定位,随后将ARRB2作为支架分子参与其中,从而激活ERK1/2通路,抑制吉西他滨诱导的细胞凋亡。此外,GPR54激动剂KP10与REG3A联合可协同增强吉西他滨耐药性,而干扰GPR54或其拮抗剂KP234可减弱REG3A诱导的吉西他滨耐药性。靶向REG3A或药理学抑制其下游信号分子可能是克服吉西他滨耐药的一种有希望的策略。总之,REG3A可以作为前列腺癌耐药的潜在生物标志物,为治疗干预和患者分层提供新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
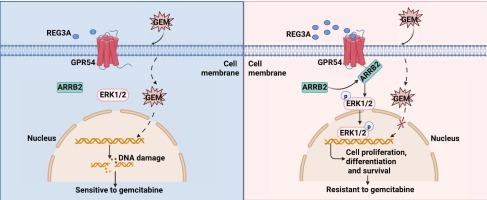
REG3A promotes gemcitabine resistance in pancreatic cancer via the GPR54/ARRB2/ERK1/2 ligand-directed signaling pathway
Gemcitabine is a widely employed first-line chemotherapeutic drug for pancreatic cancer (PCa). However, the rapid emergence of gemcitabine resistance poses a major clinical hurdle. Here, we identified REG3A as a crucial determinant of poor prognosis in PCa. Notably, REG3A was upregulated in tumor tissues from clinical gemcitabine-resistant PCa patients. Through comprehensive in vitro cellular assays and in vivo studies using Reg3g (the murine homolog of REG3A) knockout mice, we demonstrated that REG3A deficiency sensitized PCa to gemcitabine treatment. Mechanistically, RNA sequencing (RNA-Seq) combined with Protein-Protein Interaction (PPI) analysis revealed that REG3A functioned as an exocytosis protein binds to the novel membrane receptor GPR54. The interaction increased membrane localization of GPR54, which subsequently engaged ARRB2 as a scaffolding molecule, leading to activation of the ERK1/2 pathway and suppression of gemcitabine-induced apoptosis. Moreover, the GPR54 agonist KP10 synergistically enhanced gemcitabine resistance in conjunction with REG3A, while interference with GPR54 or its antagonist KP234 attenuated REG3A-induced gemcitabine resistance. Targeting REG3A or pharmacologically inhibiting its downstream signaling molecules may represent a promising strategy to overcome gemcitabine resistance. Overall, REG3A could serve as a potential biomarker for gemcitabine resistance in PCa, offering a new avenue for therapeutic intervention and patient stratification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


