慢性应激小鼠的社交恐惧消退受损:对炎症性脑标志物的影响和催产素的贡献。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
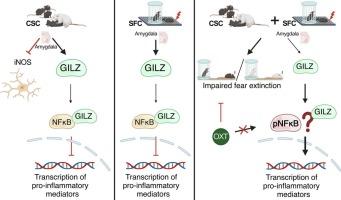
慢性社会心理压力是现代社会中常见的负担,也是许多躯体和情感障碍(包括社交焦虑症)的危险因素。长时间的压力暴露后的创伤经历通常会引发这些疾病。尽管人类和动物研究支持过度反应性免疫系统在精神病理学发病机制中起关键作用的假设,但其潜在机制尚未完全了解。因此,缺乏以免疫为重点的治疗方案。本研究在雄性C57BL/6和CD1小鼠中进行,使用慢性从属群体住房(CSC)(慢性心理社会应激小鼠模型)和社会恐惧条件反射(SFC) (SAD小鼠模型)的组合。我们可以证明,在SFC暴露之前的CSC促进了创伤性社交回避的表现,并损害了其消退,同时增加了大脑中炎症因子的释放,尤其是杏仁核。神经肽催产素(OXT)具有深刻的亲社会、压力缓冲和抗炎作用,被认为是治疗包括SAD在内的压力相关疾病的有希望的治疗选择。在这里,我们可以证明中枢OXT输注可以防止观察到的行为表型,而杏仁核的炎症参数保持不变。虽然需要进一步的机制研究,但我们的研究结果表明,慢性社会心理压力加剧了由创伤性社会事件引起的sad样症状的发展,并损害了其恢复。此外,我们的数据为OXT的应激和创伤保护作用提供了进一步的证据,但没有证实其抗炎特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Impaired social fear extinction in chronically stressed mice: Impact on inflammatory brain markers and the contribution of oxytocin
Chronic psychosocial stress is a frequent burden in modern societies and risk factor for numerous somatic and affective disorders, including social anxiety disorder (SAD). Traumatic experiences after prolonged periods of stress exposure often trigger these diseases. Although human and animal studies support the hypothesis of an over-reactive immune system being critically involved in the pathogenesis of psychopathologies, the underlying mechanisms are not fully understood. Accordingly, immune-focused treatment options are lacking.
The current study was performed in male C57BL/6 and CD1 mice using a combination of chronic subordinate colony housing (CSC), a mouse model for chronic psychosocial stress, and social fear conditioning (SFC), a mouse model for SAD. We can show that CSC prior to SFC exposure facilitates the manifestation of trauma-induced social avoidance and impairs its extinction, while increasing the release of inflammatory factors in the brain, especially in the amygdala. The neuropeptide oxytocin (OXT) with its profound pro-social, stress-buffering and anti-inflammatory effects has been suggested as a promising therapeutic option for stress-related diseases including SAD. Here, we can show that central OXT infusion protected against the observed behavioral phenotype, whereas the inflammatory parameters in the amygdala remained unchanged.
Although further mechanistic studies are warranted, our findings indicate that chronic psychosocial stress aggravates the development of SAD-like symptoms caused by a traumatic social event and impairs its recovery. In addition, our data provide further evidence for stress- and trauma-protective effects of OXT but did not confirm its anti-inflammatory properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


