五环三萜亚洲酸衍生物Sp1抑制剂的设计、合成及抗肿瘤活性研究。
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
以亚洲酸(AA)为先导化合物,设计合成了22个特异性蛋白1 (Sp1)抑制剂,分别在AA的A环和C-28位点进行了修饰,并通过HRMS、1H NMR和13C NMR对其结构进行了证实。采用四甲基唑盐(3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四氮唑,MTT)比色法测定亚洲酸衍生物对人乳腺癌细胞(MCF-7)和宫颈癌细胞(Hela)的生长抑制作用。结果表明,这些化合物均能抑制HeLa和MCF-7细胞的增殖,且衍生物均表现出比AA更强的肿瘤细胞毒性,其中化合物I8、II6和III3与阳性对照药物顺铂相当。Western blot (WB)和Cellular thermal shift assay (CETSA)分析显示,化合物I8可直接与Sp1结合,并能剂量依赖性地降低Sp1蛋白水平,提示化合物I8可能通过与Sp1结合发挥抗肿瘤作用。这为天然五环三萜亚洲酸成为一种新的抗肿瘤候选药物提供了实验基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
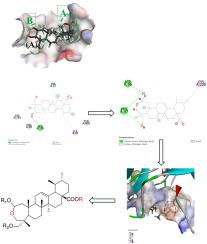
Design, synthesis and antitumor activity of pentacyclic triterpenoid Asiatic acid derivatives as Sp1 inhibitors
Asiatic acid (AA) was used as the lead compound and 22 inhibitors of specificity protein 1 (Sp1) were designed and synthesized with modification at A ring and C-28 position of AA, whose structures were confirmed by HRMS, 1H NMR and 13C NMR. The growth inhibitory effects of Asiatic acid derivatives on human breast cancer cells (MCF-7) and cervical cancer cells (Hela) were determined by tetramethyl azole salt (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, MTT) colorimetric assay. The results showed that all of these compounds inhibited the proliferation of HeLa and MCF-7 cells, and all the derivatives showed stronger tumor cytotoxicity than AA, among which compounds I8, II6, and III3 were comparable to the positive control drug cisplatin. Western blot (WB) and Cellular thermal shift assay (CETSA) assay analyses revealed that compound I8 could directly bind to Sp1 and dose-dependently reduce Sp1 protein levels, suggesting that compound I8 may exert its antitumor effects through binding to Sp1. This provides an experimental basis for the natural pentacyclic triterpenoid Asiatic acid to become a novel anti-tumor new drug candidate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


