冬凌草素通过靶向KEAP1/NRF2信号通路调节线粒体完整性和铁上塌,减轻细菌性肺炎。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
耐甲氧西林金黄色葡萄球菌(MRSA)是一种高毒力和耐药病原体,经常引起细菌性肺炎。目前,由于细菌的耐药性迅速发展,有效的治疗方法有限。因此,迫切需要开发专注于宿主-病原体相互作用的新疗法。oriidonin是一种天然存在的二萜类化合物,具有多种药理作用,但其在细菌性肺炎中的治疗潜力及其作用方式在很大程度上仍然未知。在这里,我们证明了oriidonin对MRSA肺炎具有保护作用。巨噬细胞是抗呼吸道感染的主要先天免疫细胞,经冬凌草素治疗后,巨噬细胞表现出增强的杀菌能力、减轻的炎症反应和对铁下垂的抗性。重要的是,我们进一步发现冬甲草苷与kelch样ech相关蛋白1 (KEAP1)共价结合,阻碍其与核因子红系2相关因子2 (NRF2)的结合。NRF2激活的增强随后激活了负责线粒体脂质过氧化和铁稳态的基因,从而协调肺泡巨噬细胞的活性和存活。总的来说,我们提出了第一个证据,证明了oridonin在对抗耐药细菌性肺炎方面的治疗潜力,并将其确定为线粒体和铁致凋亡途径的新型调节剂。这可能对开发针对强大病原体的宿主定向疗法具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
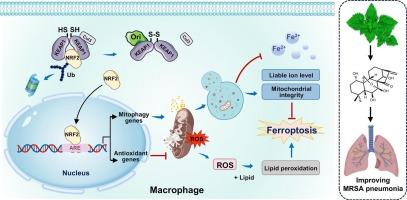
Oridonin mitigates bacterial pneumonia by regulating mitochondrial integrity and ferroptosis via targeting KEAP1/NRF2 signaling
Methicillin-resistant Staphylococcus aureus (MRSA) is a highly virulent and drug-resistant pathogen frequently causing bacterial pneumonia. Currently, there are limited effective treatments available due to the rapidly evolving resistance of bacteria. Therefore, there is an urgent need to develop novel therapies that focus on host-pathogen interactions. Oridonin is a naturally occurring diterpenoid with multiple pharmacological effects, but its therapeutic potential in bacterial pneumonia, as well as its action mode, remains largely unknown. Here, we demonstrated that oridonin conferred protection against MRSA pneumonia. Macrophages, the major innate immune cells against respiratory infection, exhibited enhanced bactericidal capability, alleviated inflammatory response, and resistance to ferroptosis upon oridonin treatment. Importantly, we further showed that oridonin covalently associates with the Kelch-like ECH-associated protein 1 (KEAP1), hindering its binding by nuclear factor erythroid 2-related factor 2 (NRF2). Enhanced activation of NRF2 subsequently activated the genes responsible for mitochondrial lipid peroxidation and iron homeostasis, thereby orchestrating the activity and survival of alveolar macrophages. Collectively, we present the first evidence demonstrating the therapeutic potential of oridonin in combating drug-resistant bacterial pneumonia, establishing it as a novel regulator of both mitochondrial and ferroptotic pathways. This may have significant implications for the development of host-directed therapies against formidable pathogens.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


