一锅绿色生物辅助合成ZnO/SnO2-rGO杂化纳米复合材料
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
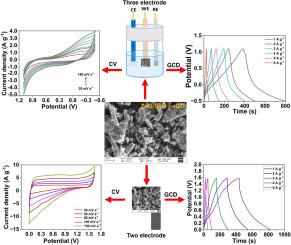
系统地研究了还原氧化石墨烯纳米片(rGO Ns)、ZnO/SnO2纳米复合材料(ZnO/SnO2 NCs)和ZnO/SnO2-rGO杂化纳米复合材料(ZnO/SnO2-rGO HNCs)的超级电容器(SC)性能。采用一锅绿色合成法,以豆豆叶提取物(PJLE)为绿色还原剂,制备了rGO纳米粒子、ZnO/SnO2纳米粒子和ZnO/SnO2-rGO纳米粒子。通过FTIR、UV、SEM、XRD、DLS、XPS等手段对合成的样品进行了分析。粉末XRD证实了ZnO/SnO2 NCs和ZnO/SnO2- rgo HNCs中存在六方纤锌矿结构的ZnO和四方SnO2纳米颗粒。SEM分析进一步揭示了ZnO纳米棒和球形SnO2纳米颗粒在还原氧化石墨烯纳米片表面的成功集成。采用循环伏安法(CV)、电化学阻抗谱法(EIS)和恒流充放电(GCD)技术研究了石墨电极(GE)修饰ZnO/SnO2 NCs和ZnO/SnO2- rgo HNCs在酸性电解质(1 M H2SO4)中的电化学性能。ZnO/SnO2-rGO修饰的GE在1 M H2SO4中具有128.43 F/g的比电容(SC),在1 a /g条件下,经过1万次GCD循环后电容保持率为95.97%。此外,所构建的对称器件(ZnO/SnO2-rGO//ZnO/SnO2-rGO HNCs)在1m H2SO4中具有165.91 F/g的高SC, 10,000 GCD循环的长寿命周期,以及212.36 Wh/kg和1440 W/kg的高能量密度(ED)和功率密度(PD)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
One-pot green bio-assisted synthesis of ZnO/SnO2-rGO hybrid nanocomposites using Prosopis juliflora leaf extract for high-performance supercapacitor electrode
The supercapacitor (SC) performance of reduced graphene oxide nanosheets (rGO Ns), ZnO/SnO2 nanocomposites (ZnO/SnO2 NCs), and ZnO/SnO2-rGO hybrid nanocomposites (ZnO/SnO2-rGO HNCs) was systematically investigated and reported. A one-pot green synthesis method was adopted for the preparation of rGO Ns, ZnO/SnO2 NCs, and ZnO/SnO2-rGO HNCs using Prosopis juliflora leaf extract (PJLE) as a green reducing agent. The synthesized samples were analyzed via FTIR, UV, SEM, XRD, DLS, and XPS. Powder XRD confirmed the existence of hexagonal wurtzite-structured ZnO and tetragonal SnO2 nanoparticles in the ZnO/SnO2 NCs and ZnO/SnO2-rGO HNCs. SEM analysis further revealed the successful integration of ZnO nanorods and spherical SnO2 nanoparticles onto the rGO nanosheet surface. The electrochemical performance of the graphite electrode (GE)-modified ZnO/SnO2 NCs and ZnO/SnO2-rGO HNCs was investigated in an acidic electrolyte (1 M H2SO4) via cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge discharge (GCD) techniques. The ZnO/SnO2-rGO HNCs-modified GE demonstrated a greater specific capacitance (SC) of 128.43 F/g in 1 M H2SO4, with a capacitance retention of 95.97 % at 1 A/g after 10,000 GCD cycles. Additionally, the constructed symmetric device (ZnO/SnO2-rGO//ZnO/SnO2-rGO HNCs) has a high SC of 165.91 F/g, a long-life cycle of 10,000 GCD cycles, and a high energy density (ED) and power density (PD) of 212.36 Wh/kg and 1440 W/kg, respectively, in 1 M H2SO4.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


