生物源氧化镍纳米颗粒用于硝基苯衍生物的催化还原及其抗癌和抗氧化活性
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
随着对环境可持续性的日益关注,绿色合成方法因其简单和环保的性质而越来越受到人们的认可。本研究提出了一种从蓖麻叶水提物中合成氧化镍纳米颗粒的创新方法。使用各种技术对合成的纳米颗粒进行了广泛的表征。x射线衍射(XRD)证实了纳米颗粒的结晶性质,平均尺寸为6.35 nm,扫描电子显微镜(SEM)和透射电子显微镜(TEM)显示了纳米颗粒的球形形貌。傅里叶变换红外光谱(FTIR)表明植物化学物质参与了纳米颗粒的合成和稳定。紫外可见光谱(UV-Vis)在270 nm处有明显的吸收。zeta电位为-19.8 mV时,表明纳米颗粒具有中等的胶体稳定性;PDI为0.390时,表明纳米颗粒的粒径分布较为均匀。通过x射线光电子能谱(XPS)和能量色散x射线(EDX)技术确定了它们的元素组成。在可持续的反应条件下,纳米颗粒在将有害硝基苯化合物还原为相应的苯胺衍生物方面表现出良好的催化活性。反应时间短,产率高。该纳米催化剂具有良好的稳定性,可连续使用5次。此外,纳米颗粒对人肺癌细胞系A549表现出明显的抗肿瘤活性,在250µg/mL浓度下,细胞毒性为84.57%,IC50值为53.16µg/mL。纳米粒子还显示出剂量依赖性的自由基清除活性,在200µg/mL浓度下,其最大清除率为73.38%。这项工作促进了绿色纳米技术的发展,并突出了蓖麻为基础的NiO纳米颗粒在催化和生物医学应用中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
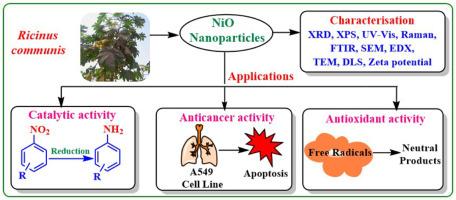
Biogenic nickel oxide nanoparticles for the catalytic reduction of nitrobenzene derivatives and their anticancer and antioxidant activities
With the increasing focus on environmental sustainability, green synthesis methods are being increasingly recognised for their simplicity and eco-friendly nature. This study presents an innovative approach for synthesising nickel oxide (NiO) nanoparticles from the aqueous leaf extract of Ricinus communis. The synthesised nanoparticles were extensively characterised using various techniques. X-ray Diffraction (XRD) confirmed the crystalline nature of the nanoparticles with an average size of 6.35 nm, while Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) revealed their spherical morphology. The Fourier Transform Infrared Spectroscopy (FTIR) indicated the involvement of phytochemicals in the synthesis and stabilisation of the nanoparticles. The UV–Visible Spectroscopy (UV–Vis) showed a distinct absorption at 270 nm. The zeta potential of -19.8 mV showed moderate colloidal stability, and a polydispersity index (PDI) of 0.390 suggested a moderately uniform size distribution of the nanoparticles. Their elemental composition was confirmed through X-ray Photoelectron Spectroscopy (XPS) and Energy Dispersive X-ray (EDX) technique. The nanoparticles demonstrated promising catalytic activity in the reduction of hazardous nitrobenzene compounds to their corresponding aniline derivatives under sustainable reaction conditions. The reactions gave a good yield of products in brief reaction times. The nanocatalyst exhibited good stability and was effectively reusable for up to five consecutive cycles. Moreover, the nanoparticles showed significant anticancer activity against the A549 human lung cancer cell line, with 84.57 % cytotoxicity at 250 µg/mL concentration and an observed IC50 value of 53.16 µg/mL. The nanoparticles also displayed a dose-dependent free radical scavenging activity, with a maximum scavenging of 73.38 % found at 200 µg/mL concentration. This work contributes to the advancement of green nanotechnology and highlights the potential of Ricinus communis-based NiO nanoparticles in catalytic and biomedical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


