NR1I3通过增强pck1介导的糖异生抑制结直肠癌的生长
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
越来越多的证据表明,核受体亚家族1I组成员3 (NR1I3)在许多恶性肿瘤的进展中起着重要作用。然而,NR1I3是否抑制结直肠癌(CRC)生长或改变糖异生尚不清楚。采用Western blotting、流式细胞术分析、细胞增殖、集落形成实验、定量实时聚合酶链反应(qRT-PCR)、糖异生实验和动物模型研究NR1I3在结直肠癌细胞中的功能作用。我们发现NR1I3在结直肠癌组织中经常下调,NR1I3的低表达与患者生存不良密切相关。随后的体外和体内功能试验表明,NR1I3通过将细胞周期阻滞在G2/M期,显著抑制CRC细胞的增殖和诱导凋亡。我们还发现,6-(4-氯苯基)咪唑[2,1-b][1,3]噻唑-5-羧基-(3,4-二氯苯基)肟(CITCO)在体外和体内诱导NR1I3可降低CRC细胞生长并诱导凋亡。此外,我们证明了CITCO可以通过影响糖异生途径中的基因来影响糖异生活性。值得注意的是,NR1I3通过与限制糖异生速率的磷酸烯醇丙酮酸羧激酶1 (PCK1)相互作用,增加糖异生并抑制糖酵解。这导致ATP耗竭,细胞生长停止。这些发现表明NR1I3通过PCK1将糖酵解转化为糖异生来抑制CRC,提示CRC进展的潜在指标和治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
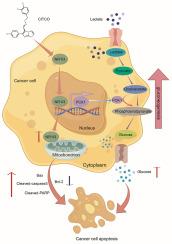
NR1I3 inhibits colorectal cancer growth by enhancing PCK1-mediated gluconeogenesis
There is increasing evidence that nuclear receptor subfamily 1 group I member 3 (NR1I3) plays a significant role in the progression of many malignancies. However, it is unclear whether NR1I3 suppresses colorectal cancer (CRC) growth or alters gluconeogenesis. Western blotting, flow cytometry analysis, cell proliferation, colony formation assays, quantitative real-time polymerase chain reaction (qRT‒PCR), gluconeogenesis tests, and animal models were used to examine the functional role of NR1I3 in CRC cells. We found that NR1I3 was frequently downregulated in CRC tissue and that low NR1I3 expression was strongly correlated with poor patient survival. Subsequent in vitro and in vivo functional tests demonstrated that NR1I3 significantly inhibited proliferation and induced apoptosis in CRC cells by arresting the cell cycle in the G2/M phase. We also found that pharmacologically inducing NR1I3 with 6-(4-chlorophenyl) imidazo[2,1-b][1,3] thiazole-5-carbaldehydeO-(3,4-dichlorobenzyl) oxime (CITCO) reduced CRC cell growth and induced apoptosis in vitro and in vivo. Furthermore, we demonstrated that CITCO can influence gluconeogenesis activity by influencing genes in the gluconeogenesis pathway. Notably, NR1I3 increases gluconeogenesis and inhibits glycolysis by interacting with phosphoenolpyruvate carboxykinase 1 (PCK1), the enzyme that limits the rate of gluconeogenesis. This leads to ATP depletion, and cell growth is halted. These findings suggest that NR1I3 inhibits CRC by converting glycolysis to gluconeogenesis via PCK1, suggesting potential indicators and treatment targets for CRC progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


