具有有效抗增殖活性的多靶点三嗪吲哚衍生物的设计和合成:靶向EGFR, SIRT1, MDM2, Hsp90, PI3K, p53和caspase-9
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
由于迫切需要能够克服传统治疗方法局限性的新型抗癌药物,设计、合成了一系列具有柔性吡唑或吡唑啉基团的新型苯基- 5h -[1,2,4]三氮基[5,6-b]吲哚衍生物,并对其抗增殖效果进行了评估。与对照化合物INZ相比,大多数合成的化合物显示出显著的细胞毒性。其中,化合物4、10、11和12对A549肺癌细胞具有较强的抑制活性,IC50值分别为7.39 μM、3.17 μM、0.82 μM和5.38 μM,优于INZ (IC50 = 8.97 μM)。化合物4、5和10对Caco-2结直肠癌细胞的IC50值分别为2.35 μM、3.28 μM和2.63 μM,而INZ的IC50值为3.64 μM。为了阐明大多数活性化合物抗癌活性的潜在机制,我们进行了一套全面的实验,包括细胞周期分析、细胞凋亡评估和关键分子靶点如EGFR、SIRT1、MDM2、Hsp90、PI3K、p53和caspase-9的量化。这些生物标志物在细胞信号通路中错综复杂地相互关联,因此抑制EGFR和PI3K抑制增殖信号,下调SIRT1和MDM2导致p53激活,随后诱导caspase-9介导的凋亡和细胞周期阻滞。值得注意的是,大多数被测试的化合物在调节这些靶标方面显示出有希望的结果,这支持了它们作为有效抗癌剂的潜力。此外,选定的化合物在停靠到EGFR、Hsp90、PI3K、SIRT1和MDM2的活性区域时,显示出与目标位点的良好结合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
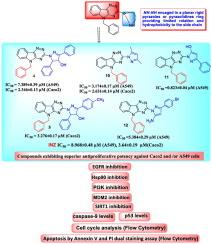
Design and synthesis of multitarget triazinoindole derivatives with potent antiproliferative activity: Targeting EGFR, SIRT1, MDM2, Hsp90, PI3K, p53, and caspase-9
Driven by the urgent need for novel anticancer agents capable of overcoming limitations associated with conventional therapies, a new series of Benzyl-5H-[1,2,4]triazino[5,6-b]indoles derivatives bearing flexible pyrazole or pyrazoline moieties were designed, synthesized, and evaluated for antiproliferative efficacy. Most of the synthesized compounds demonstrated notable cytotoxicity compared to the reference compound inauhzin (INZ). Specifically, compounds 4, 10, 11, and 12 exhibited potent activity against A549 lung cancer cells, with IC50 values of 7.39 μM, 3.17 μM, 0.82 μM, and 5.38 μM, respectively, outperforming INZ (IC50 = 8.97 μM). Against Caco-2 colorectal cancer cells, compounds 4, 5, and 10 displayed IC50 values of 2.35 μM, 3.28 μM, and 2.63 μM, respectively, compared to INZ (IC50 = 3.64 μM).
To elucidate the potential mechanisms underlying the anticancer activity of the most active compounds, a comprehensive set of assays was conducted, including cell cycle analysis, apoptosis evaluation, and quantification of key molecular targets such as EGFR, SIRT1, MDM2, Hsp90, PI3K, p53, and caspase-9. These biomarkers are intricately interconnected within cellular signaling pathways, whereby inhibition of EGFR and PI3K suppresses proliferative signaling, downregulation of SIRT1 and MDM2 leads to p53 activation, and subsequent induction of caspase-9-mediated apoptosis and cell cycle arrest. Notably, most of the tested compounds demonstrated promising results in modulating these targets, which supports their potential as effective anticancer agents. Additionally, selected compounds showed excellent binding to the target sites when docked into the active domains of EGFR, Hsp90, PI3K, SIRT1, and MDM2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


