一种新的芳基萘木脂素类似物靶向CYP51抑制新型隐球菌的生长
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
JR20是一种新型芳萘木脂素衍生物,通过靶向CYP51和线粒体功能的双重机制,对新型隐球菌具有较强的抗真菌活性(IC50 = 2.82 μg/mL)。结合分子对接、转录组学和生化分析的机制研究证实了JR20与CYP51的特异性结合,显著减少麦角甾醇的生物合成并导致膜破坏(SEM/TEM显示细胞壁塌陷和质解)。流式细胞术显示真菌坏死诱导,而线粒体功能障碍通过膜电位损失、ROS积累和ATP合成损伤证明。在小鼠深部感染模型的体内研究表明,JR20具有抗感染和抗炎双重功效。作为木脂素衍生物,JR20通过其独特的双作用机制,是一种很有前景的抗真菌药物,这凸显了天然产物研究在开发抗耐药病原体的新型抗真菌药物方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
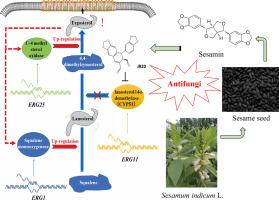
A novel arylnaphthalene lignan analogue targets CYP51 to inhibit Cryptococcus neoformans growth
JR20, a novel arylnaphthalene lignan derivative, exhibits potent antifungal activity against Cryptococcus neoformans (IC50 = 2.82 μg/mL) through dual mechanisms targeting CYP51 and mitochondrial function. Mechanistic studies combining molecular docking, transcriptomics, and biochemical assays confirmed JR20's specific binding to CYP51, significantly reducing ergosterol biosynthesis and causing membrane disruption (evidenced by SEM/TEM showing cell wall collapse and plasmolysis). Flow cytometry revealed fungal necrosis induction, while mitochondrial dysfunction was demonstrated through membrane potential loss, ROS accumulation, and ATP synthesis impairment. In vivo studies using a murine deep infection model demonstrated JR20's therapeutic efficacy, showing both anti-infective and anti-inflammatory properties. As a lignan derivative, JR20 represents a promising antifungal candidate addressing drug resistance through its unique dual-action mechanism, highlighting the potential of natural product research for developing novel antifungals against resistant pathogens.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


