一种新型磷酸肌肽3-激酶γ (PI3Kγ)抑制剂的鉴定和优化,作为一种潜在的乳腺癌抗癌药物
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
PI3K/Akt/mTOR信号通路是控制细胞生长、代谢和存活的关键调控因子,其异常激活与包括乳腺癌在内的多种恶性肿瘤的发生和预后不良密切相关。鉴于其在乳腺癌发病机制中的突出作用,这一途径代表了治疗干预的一个引人注目的目标。在本研究中,采用虚拟配体筛选方法来鉴定新的PI3Kγ抑制剂,从而选择了6种潜在化合物(L1-L6),其中L6在PI3Kγ活性位点表现出最高的结合亲和力和稳定性。分子对接分析表明,L6与关键氨基酸残基(特别是glu880)的相互作用与其他已知的类黄酮抑制剂相似,表明其结合机制保守。随后的生物学评价显示,L6显著抑制MCF7乳腺癌细胞的增殖,IC50为3.9µM。机制研究表明,L6通过下调CDK1和Cyclin B1诱导细胞周期阻滞在G2/M期,并通过激活caspase-3促进细胞凋亡。此外,L6抑制了促转移基因S100A4和MMP-9的表达,进一步支持了其抗癌潜力。药代动力学分析也显示L6具有良好的ADMET特性,表明其具有良好的药物相似性和安全性。这些发现突出了L6作为一种具有结构和功能优势的有前途的PI3Kγ抑制剂。其抗增殖、促凋亡和基因抑制作用支持其在乳腺癌治疗中的潜力,并保证其作为下一代抗癌药物的进一步发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
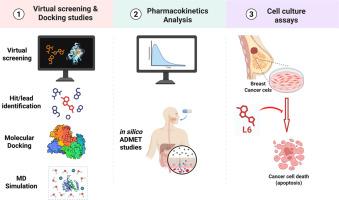
Identification and optimization of a novel phosphoinositide 3-kinaseγ (PI3Kγ) inhibitor as a potential anticancer agent against breast cancer
The PI3K/Akt/mTOR signaling pathway serves as a key regulator in controlling cell growth, metabolism, and survival, and its abnormal activation is strongly implicated in tumorigenesis and poor prognosis of various malignancies, including breast cancer. Given its prominence in breast cancer pathogenesis, this pathway represents a compelling target for therapeutic intervention. In the present study, a virtual ligand screening approach was employed to identify novel PI3Kγ inhibitors, leading to the selection of six potential compounds (L1–L6), with L6 showing the highest binding affinity and stability in the PI3Kγ active site. Molecular docking analysis revealed that L6 interacts with key amino acid residues, notably Glu-880, similarly to other known flavonoid inhibitors, suggesting a conserved binding mechanism. Subsequent biological evaluation showed that L6 significantly inhibited proliferation of MCF7 breast cancer cells with an IC50 of 3.9 µM. Mechanistic studies indicated that L6 induces cell cycle arrest at the G2/M phase by downregulating CDK1 and Cyclin B1 and promotes apoptosis via activation of caspase-3. Furthermore, L6 suppressed the expression of pro-metastatic genes S100A4 and MMP-9, further supporting its anticancer potential. The pharmacokinetic analysis also revealed favourable ADMET properties for L6, suggesting good drug-likeness and safety. These findings highlight L6 as a promising PI3Kγ inhibitor with structural and functional advantages. Its antiproliferative, pro-apoptotic, and gene-suppressive effects support its potential in breast cancer therapy and warrant further development as a next-generation anticancer agent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


