有机催化双迈克尔加成法对映选择性全合成(+)-Lucidumone
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以市售的2-环己烯-1- 1为原料,通过10步(LLS)完成了(+)-lucidumone(一种具有双环[2.2.2]辛烷值骨架的笼状多环甲萜类化合物)的简明对映选择性全合成。关键步骤是奎宁衍生物催化的双Michael加成,构建具有五个连续立体中心的必需手性双环[2.2.2]辛烷值框架。其他功能包括Nef反应和一锅Brønsted酸促进环化,同时形成茚和氢呋喃环体系。本文章由计算机程序翻译,如有差异,请以英文原文为准。

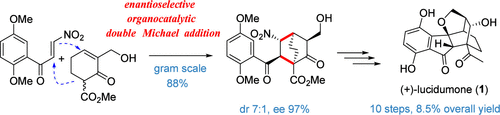
Enantioselective Total Synthesis of (+)-Lucidumone via Organocatalytic Double Michael Addition
A concise enantioselective total synthesis of (+)-lucidumone, a caged polycyclic meroterpenoid with a bicyclo[2.2.2]octane skeleton, was accomplished in 10 steps (LLS) starting from commercially available 2-cyclohexen-1-one. The key step features a quinine derivative-catalyzed double Michael addition that constructs the essential chiral bicyclo[2.2.2]octane framework with five contiguous stereocenters. Additional features include a Nef reaction and a one-pot Brønsted acid-promoted cyclization, which simultaneously form both Indane and hydrofuran ring systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


