肿瘤抗原PRAME通过SIRT1-DBC1轴使p53失活,从而促进黑色素瘤生长。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
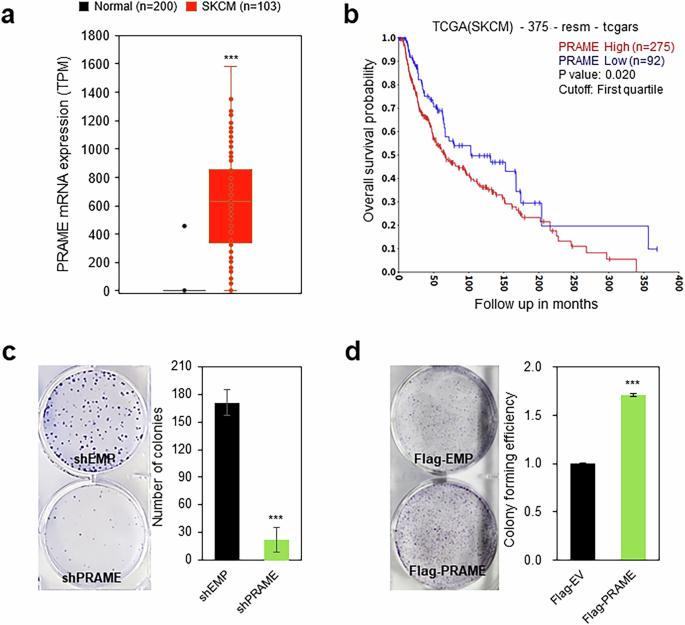
黑色素瘤中优先表达抗原(PRAME)在黑色素瘤中高度表达,与肿瘤进展和恶性相关。值得注意的是,黑色素瘤细胞尽管携带野生型p53基因,但经常表现出肿瘤抑制因子p53的失活。在这里,我们研究了PRAME和p53之间的功能相互作用。与我们对人类数据库的分析一致,PRAME过表达促进黑色素瘤细胞增殖。相反,PRAME下调会产生相反的效果,并伴有细胞凋亡的增加。RNA测序显示PRAME缺失后p53靶基因的异常调控,逆转录-定量聚合酶链反应和荧光素酶报告基因试验进一步支持了这一结论。为了探索潜在的机制,我们分离了PRAME蛋白复合物,并鉴定了SIRT1抑制因子DBC1作为该复合物的一个组成部分。此外,我们观察到PRAME促进p53去乙酰化。PRAME与DBC1的相互作用释放出DBC1中的SIRT1,从而激活SIRT1和随后的p53去乙酰化。裸鼠异种移植分析表明,PRAME缺失和SIRT1抑制联合使用可显著促进黑色素瘤细胞的生长迟缓。总之,这些发现表明,在黑色素瘤发病过程中获得的PRAME水平升高可能抑制p53通路,从而促进肿瘤生长。我们认为PRAME沉默联合SIRT1抑制剂是一种很有前景的黑色素瘤治疗策略,可以恢复p53活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tumor antigen PRAME promotes melanoma growth by inactivating p53 through the SIRT1-DBC1 axis
Preferentially expressed antigen in melanoma (PRAME), which is highly expressed in melanoma, is associated with tumor progression and malignancy. Notably, melanoma cells often exhibit inactivation of the tumor suppressor p53 despite carrying the wild-type p53 gene. Here, we investigated the functional interplay between PRAME and p53. Consistent with our analysis of human databases, PRAME overexpression promoted melanoma cell proliferation. Conversely, PRAME downregulation produced the opposite effects, accompanied by an increase in apoptosis. RNA sequencing revealed aberrant regulation of p53 target genes following PRAME depletion, which was further supported by reverse transcription-quantitative polymerase chain reaction and luciferase reporter assays. To explore the underlying mechanism, we isolated the PRAME protein complex and identified DBC1, an SIRT1 suppressor, as a component of the complex. Furthermore, we observed that PRAME promoted p53 deacetylation. The interaction of PRAME with DBC1 releases SIRT1 from DBC1, enabling SIRT1 activation and subsequent p53 deacetylation. The combination of PRAME depletion and SIRT1 inhibition can significantly promote the growth retardation of melanoma cells, as demonstrated by xenograft analysis in nude mice. Collectively, these findings suggest that the acquired elevation of the PRAME level during melanoma pathogenesis may suppress p53 pathways, thereby promoting tumor growth. We propose that PRAME silencing combined with the use of SIRT1 inhibitors is a promising therapeutic strategy for melanoma by restoring p53 activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


