新型多功能化吡啶衍生物对库蚊的合成、杀幼虫活性及其计算机机制研究。
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
以2-氨基-4-(4-氯苯基)-6-(对苯基)烟腈(M)为核心,通过高效的多组分和环化策略,开发了一个具有战略设计和生态意识的合成平台,以获取18个多功能化吡啶基杂环化合物的新文库。通过常规和加速(微波/超声辅助)途径整合强效药效团,包括吡啶[2,3-d]嘧啶、萘嘧啶、三嗪和融合吡罗/四唑基序,实现了结构多样性的最大化,并通过IR、1H/13C NMR和质谱证实,获得了具有高结构保真度的优异产率。生物学评价显示,所有合成的化合物对库蚊幼虫具有优异的杀幼虫功效,特别是15和9,成为主要候选物质,其LC₅0值分别为0.118和0.137 μg/mL。最有效化合物的生化分析表明,对关键解毒酶,乙酰胆碱酯酶,细胞色素P450单加氧酶和羧酸酯酶有明显的抑制作用。先进的硅分析,包括分子对接、分子动力学(200 ns)、MM/GBSA结合自由能和ADMET预测,阐明了结合稳定性、机制途径和良好的生态安全特征,包括低预测的环境持久性。总的来说,这些结果表明,合成的衍生物,特别是化合物15,作为有希望的一代具有双重作用的杀幼虫剂,以一种或多种作用方式为目标,以减少抗药性的产生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
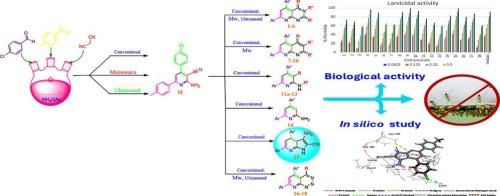
Synthesis, Larvicidal Activity, and In Silico Mechanistic Insights of Novel Polyfunctionalized Pyridine Derivatives Against Culex pipiens L.
A strategically engineered, eco-conscious synthetic platform was developed to access a novel library of eighteen polyfunctionalized pyridine-based heterocycles through high-efficiency multicomponent and annulation strategies, using 2-amino-4-(4-chlorophenyl)-6-(p-tolyl)nicotinonitrile (M) as a privileged core. Structural diversity was maximized by integrating potent pharmacophores, including pyrido[2,3-d]pyrimidines, naphthyridines, triazines, and fused pyrrolo/tetrazolo motifs, via both conventional and accelerated (microwave/ultrasound-assisted) routes, affording excellent yields with high structural fidelity as confirmed by IR, 1H/13C NMR, and mass spectrometry. Biological evaluation revealed that all synthesized compounds had excellent larvicidal efficacy against Culex pipiens larvae, especially 15 and 9, emerging as lead candidates that exhibited exceptional LC₅₀ values of 0.118 and 0.137 μg/mL, respectively. Biochemical assays of the most effective compounds demonstrated pronounced inhibition of key detoxification enzymes, acetylcholinesterase, cytochrome P450 monooxygenase, and carboxylesterase. Advanced in silico analyses, encompassing molecular docking, molecular dynamics (200 ns), MM/GBSA binding free energy, and ADMET predictions, elucidated binding stability, mechanistic pathways, and favorable eco-safety profiles, including low predicted environmental persistence. Collectively, these results position the synthesized derivatives, particularly compound 15, as a promising generation of larvicidal agents with dual action targets one or more modes of action to reduce the development of resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


