甲基萘醌系于1h -1,2,3-三唑基硒酸酯的抗真菌特性:合成、体外和硅分析
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
侵袭性念珠菌感染是一个临床挑战,有效的治疗药物有限,耐药性增加。迫切需要发现新的抗真菌药物。本研究以h -1,2,3-三唑基硒酸酯为链结物,开发了一系列具有抗假丝酵母菌活性的2-甲基-1,4-萘醌(Menadione)。研究了21个化合物对念珠菌的体外抗真菌和抗膜活性,并在不同浓度下对几种念珠菌均有抑制作用。两种化合物对光秃假丝酵母浮游细胞和生物膜的抑菌活性分别为16和32 μg/mL。通过计算机研究确定了抗真菌蛋白靶点与BonFIRE光谱图像的结合能,并提供了立体电子学、药代动力学和毒理学图谱。通过与CYP51和Hsp90的分子对接分析,与伊曲康唑和radicicol相比,突出了化合物的抑制潜力,并进一步强调了所有分子具有良好的药代动力学特征,其特点是毒性低。因此,我们得出结论,其中两种化合物对光秃葡萄球菌具有较强的抗真菌活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
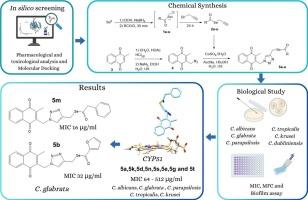
Antifungal profile of menadione tethered to 1H-1,2,3-triazolyl-selenoester: synthesis, in vitro and in silico analyses
Invasive Candidiasis infections are a clinical challenge, with limited effective therapeutic agents and increasing resistance. The discovery of new antifungal agents is urgently required. Here, we developed a new series of 2-methyl-1,4-naphthoquinone (Menadione) Tethered to 1H-1,2,3-triazolyl-selenoester in good yields, which exhibit antifungal potential activity against Candida species. Antifungal and antibiofilm activities for the twenty-one compounds were performed in vitro against Candida spp and exhibited inhibitory effects at different concentrations for several Candida species. Two compounds showed strong inhibitory effects, with fungicidal activity at 16 and 32 μg/mL, respectively, against planktonic cells and biofilms of Candida glabrata. In silico studies were conducted to determine the binding energies against BonFIRE spectral images of antifungal protein targets, and provided stereo electronic, pharmacokinetic and toxicological profiles. The analysis through molecular docking with CYP51 and Hsp90 highlighted the inhibitory potential of the compounds in comparison to itraconazole and radicicol, and further underscored the favorable pharmacokinetic profile for all molecules, characterized by a low toxicity profile. We therefore concluded that two compounds especially exhibited potent antifungal activity against C. glabrata.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


