胰岛素诱导的AKT泛素化需要依赖importin7的PDK1核转运
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
AKT是参与多种细胞过程的关键激酶,包括肿瘤发生和糖原代谢。据报道,AKT的泛素化修饰是调节其激酶活性和膜易位的关键细胞事件;然而,AKT泛素化的分子机制尚不明确。在这里,我们采用功能缺失方法和PDK1磷酸化改变和核质穿梭行为改变的突变体来确定PDK1在AKT泛素化中的功能作用。我们的研究结果表明,胰岛素诱导的traf6介导的AKT泛素化需要PDK1以依赖importin7的方式进入细胞核,这一过程先前已被证明发生在细胞核中。此外,PDK1在S241位点的磷酸化和pi3k介导的PDK1在S396位点的磷酸化都通过控制PDK1的核进入来调节AKT的泛素化。这些结果为AKT信号的控制提供了新的见解,并为开发靶向AKT调节疗法提供了潜在的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
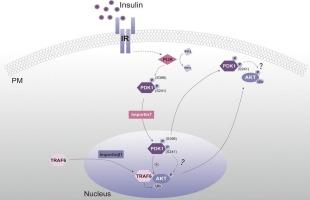
Nuclear trafficking of PDK1 in importin7-dependent manner is required for insulin-induced AKT ubiquitination
AKT is a pivotal kinase involved in diverse cellular processes, including tumorigenesis and glycogen metabolism. Ubiquitination modification of AKT has been reported as a critical cellular event that regulates its kinase activity and membrane translocation; however, the molecular mechanisms involved in AKT ubiquitination remain elusive. Here, we employed loss-of-function approaches and mutants of PDK1 with altered phosphorylation and modified nucleocytoplasmic shuttling behaviors to identify the functional roles of PDK1 on the ubiquitination of AKT. Our findings reveal that the nuclear entry of PDK1 in an importin7-dependent manner is required for insulin-induced TRAF6-mediated ubiquitination of AKT, which has been previously shown to occur in the nucleus. Moreover, the phosphorylation of PDK1 at S241 and the PI3K-mediated phosphorylation of PDK1 at S396 both regulate AKT ubiquitination by controlling nuclear entry of PDK1. These results provide new insights into the control of AKT signaling and suggest potential avenues for developing targeted AKT-modulating therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


