sigma-1受体敲除大鼠的产生和表型特征
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
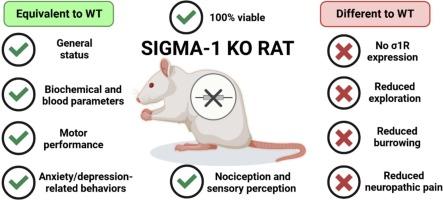
sigma-1受体(σ1R)是参与疼痛调节、神经保护和神经退行性疾病等多种生理和病理过程的伴侣。尽管其功能意义重大,但由于缺乏合适的模型进行详细的机制研究,其确切作用尚不清楚。在这项工作中,我们描述了一种新的σ1R敲除(σ1R KO)大鼠模型的产生和表型特征。利用CRISPR/Cas9技术,我们在σ1R基因中引入了218个碱基对的特异性缺失,导致受体表达完全丧失,这一点经Western blot、免疫组织化学和结合实验证实。综合表型分析显示,在基线条件下,σ1R大鼠的发育和行为没有明显异常,表明σ1R对发育和生存不是必需的。此外,在细胞或生化血液参数中没有观察到基因型相关的差异。运动功能测试(旋转杆、握力和车轮运行)显示无缺陷;然而,σ1R KO大鼠的探索行为和掘洞行为明显减少。相比之下,在开阔场地、惊吓或强迫游泳测试中没有观察到焦虑抑郁样行为。幼龄大鼠的感觉测试显示,在机械、热或冷刺激或福尔马林测试(化学诱导的疼痛)的反应中,没有显著的基因型相关差异。然而,σ1R KO大鼠在创伤性神经损伤后表现出减轻的神经性疼痛,表明σ1R在疼痛致敏通路中的作用。本研究建立了σ1R KO大鼠作为研究σ1R介导机制和开发针对σ1R治疗慢性疼痛、神经退行性疾病和精神疾病的治疗策略的有价值的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Generation and phenotypic characterization of a sigma-1 receptor knockout rat
The sigma-1 receptor (σ1R) is a chaperone involved in multiple physiological and pathological processes, including pain modulation, neuroprotection, and neurodegenerative diseases. Despite its functional significance, its precise roles remain unclear due to the lack of suitable models for detailed mechanistic studies. In this work, we describe the generation and phenotypic characterization of a novel σ1R knockout (σ1R KO) rat model. Using CRISPR/Cas9 technology, we introduced a specific 218-base-pair deletion into the σ1R gene, resulting in a complete loss of receptor expression, as confirmed by Western blot, immunohistochemistry, and binding assays. Comprehensive phenotypic analyses revealed no major developmental or behavioral abnormalities in σ1R KO rats under baseline conditions, suggesting that σ1R is not essential for development or survival. Additionally, no genotype-related differences were observed in cellular or biochemical blood parameters. Motor function tests (rotarod, grip strength, and wheel running) showed no deficits; however, σ1R KO rats displayed reduced exploratory behavior in actimetry and markedly diminished burrowing behavior. By contrast, no anxiodepressive-like behaviors were observed in the open field, startle, or forced swim tests. Sensory testing of naive rats revealed no significant genotype-related differences in responses to mechanical, heat, or cold stimuli, or in the formalin test (chemical-induced pain). However, σ1R KO rats displayed attenuated neuropathic pain after traumatic nerve injury (spared nerve injury), highlighting the role of σ1R in pain sensitization pathways. This study establishes the σ1R KO rat as a valuable tool for investigating σ1R-mediated mechanisms and for developing therapeutic strategies targeting σ1R for chronic pain, neurodegeneration, and psychiatric disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


