硫化氢通过下调CXCL12抑制胶质母细胞瘤中单核细胞源性肿瘤相关巨噬细胞的募集
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肿瘤相关巨噬细胞(Tumor associated macrophages, tam)通过阻止抗肿瘤免疫、促进肿瘤侵袭和血管生成,直接参与胶质母细胞瘤的不良预后。抑制TAM浸润是胶质母细胞瘤的一种潜在治疗策略,有几种趋化因子拮抗剂处于早期临床开发阶段。硫化氢是一种调节多种中枢神经系统疾病中小胶质细胞聚集的气体递质,可能是防止胶质母细胞瘤中TAM募集的新治疗靶点。在这项研究中,从14个异柠檬酸脱氢酶(IDH)野生型胶质母细胞瘤手术样本中直接测量硫化氢浓度,并与总生存率以及TAM标记物和趋化因子的表达进行比较。在基因工程小鼠胶质母细胞瘤模型中,硫化氢供体治疗对生存和TAM募集的影响也被检测。高硫化氢浓度与单核细胞源性TAM密度降低和CXCL12下调相关,可使idh野生型胶质母细胞瘤患者的生存获益。通过将硫化氢供体SG1002给予免疫功能小鼠胶质母细胞瘤模型,这些发现得到了验证,该模型提高了小鼠存活率,抑制了单核细胞浸润,并下调了CXCL12。最后,硫化氢供体治疗直接降低了胶质母细胞瘤细胞中CXCL12的表达,降低了它们在体外募集单核细胞的能力。综上所述,这些结果表明硫化氢信号通过抑制趋化性来阻止单核细胞来源的TAM在胶质母细胞瘤中的积累。本文章由计算机程序翻译,如有差异,请以英文原文为准。
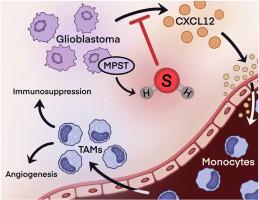
Hydrogen sulfide inhibits recruitment of monocyte-derived tumor associated macrophages in glioblastoma by downregulating CXCL12
Tumor associated macrophages (TAMs) directly contribute to the dismal prognosis of glioblastoma by preventing anti-tumor immunity and promoting tumor invasion and angiogenesis. Inhibiting TAM infiltration is a potential therapeutic strategy in glioblastoma, with several chemokine antagonists in early clinical development. Hydrogen sulfide, a gasotransmitter that regulates microglial accumulation in a wide range of CNS diseases, may be a novel therapeutic target to prevent TAM recruitment in glioblastoma. In this study, hydrogen sulfide concentrations were directly measured from 14 isocitrate dehydrogenase (IDH)-wildtype glioblastoma surgical samples and compared against overall survival as well as expression of TAM markers and chemokines. Effects of hydrogen sulfide donor therapy on survival and TAM recruitment were also examined in a genetically engineered mouse model of glioblastoma. High hydrogen sulfide concentrations conferred a survival benefit in IDH-wildtype glioblastoma, in association with reduced monocyte-derived TAM density and downregulation of CXCL12. These findings were validated by administering hydrogen sulfide donor SG1002 to an immunocompetent mouse model of glioblastoma, which improved survival, inhibited monocyte infiltration, and downregulated CXCL12. Finally, hydrogen sulfide donor treatment directly reduced CXCL12 expression in glioblastoma cells, diminishing their ability to recruit monocytes in vitro. Taken together, these results demonstrate that hydrogen sulfide signaling prevents monocyte-derived TAM accumulation in glioblastoma by inhibiting chemotaxis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


