细胞粘附分子ITGB2促进b细胞恶性肿瘤的CAR-T细胞治疗
IF 10.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
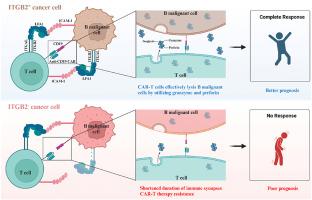
CAR-T细胞疗法作为肿瘤免疫治疗的代表技术,在血液系统恶性肿瘤的治疗中取得了显著的成功;然而,很大比例的患者未能达到持续缓解。通过对CD19 CAR-T细胞治疗前骨髓测序数据的分析,我们发现细胞粘附是影响临床结果的关键因素。我们开发了一个基于与细胞粘附相关的核心基因来预测B-ALL治疗效果的模型,并在我们的临床队列中得到了验证。体外和体内实验均显示,在恶性B细胞中抑制或敲除整合素亚单位β 2 (ITGB2,也称为CD18)可显著降低CD19和CD20 CAR-T细胞对B系肿瘤细胞的细胞毒作用,而ITGB2介导的细胞毒性改变与肿瘤微环境中免疫突触的形成有关。值得注意的是,通过LPS或Venetoclax上调Nalm6细胞中的ITGB2可显著增强CAR-T细胞对Nalm6细胞的细胞毒活性。我们的研究结果为临床CD19 CAR-T细胞治疗提供了一个新的预测模型,并阐明了ITGB2途径在CAR-T细胞介导的b细胞恶性肿瘤根除中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cell adhesion molecule ITGB2 promotes CAR-T cell therapy in B-cell malignancies
CAR-T cell therapy, as a representative technology in cancer immunotherapy, has demonstrated notable success in the treatment of hematologic malignancies; however, a significant proportion of patients fail to achieve sustained remission. Through the analysis of bone marrow sequencing data prior to CD19 CAR-T cell therapy, we identified cellular adhesion as a pivotal factor influencing clinical outcomes. We developed a model to predict B-ALL treatment efficacy based on the core genes associated with cellular adhesion, which was validated in our clinical cohort. Both in vitro and in vivo experiments revealed that the inhibition or knockout of integrin subunit beta 2 (ITGB2, also known as CD18) in malignant B cells markedly diminished the cytotoxic efficacy of both CD19 and CD20 CAR-T cells against B-lineage tumor cells, with alterations in ITGB2-mediated cytotoxicity linked to the formation of immunological synapses within the tumor microenvironment. Notably, the upregulation of ITGB2 in Nalm6 cells via LPS or Venetoclax significantly augmented the cytotoxic activity of CAR-T cells against Nalm6 cells. Our findings provide a novel predictive model for clinical CD19 CAR-T cell therapy and elucidate a role of the ITGB2 pathway in CAR-T cell-mediated eradication of B-cell malignancies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer letters
医学-肿瘤学
CiteScore
17.70
自引率
2.10%
发文量
427
审稿时长
15 days
期刊介绍:
Cancer Letters is a reputable international journal that serves as a platform for significant and original contributions in cancer research. The journal welcomes both full-length articles and Mini Reviews in the wide-ranging field of basic and translational oncology. Furthermore, it frequently presents Special Issues that shed light on current and topical areas in cancer research.
Cancer Letters is highly interested in various fundamental aspects that can cater to a diverse readership. These areas include the molecular genetics and cell biology of cancer, radiation biology, molecular pathology, hormones and cancer, viral oncology, metastasis, and chemoprevention. The journal actively focuses on experimental therapeutics, particularly the advancement of targeted therapies for personalized cancer medicine, such as metronomic chemotherapy.
By publishing groundbreaking research and promoting advancements in cancer treatments, Cancer Letters aims to actively contribute to the fight against cancer and the improvement of patient outcomes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


