PFGA12通过直接调节PRDX1,抑制铁下垂,改善缺氧缺血性脑损伤
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
缺氧缺血性脑损伤(HIBD)是一种严重的疾病,可导致广泛的神经元丧失和功能损伤,是新生儿护理的一个重大挑战。PFGA12是一种源自纤维蛋白原α链(FGA)的肽,在缺氧缺血性脑病(HIE)婴儿的脐带血中明显下调。我们证明PFGA12显著提高细胞活力,减轻氧葡萄糖剥夺/再灌注(OGD/R)诱导的神经元细胞死亡。PFGA12治疗可显著缓解HIBD大鼠脑水肿,减少梗死体积,减轻神经元损伤,这是由于PFGA12在神经元内的稳定存在。此外,PFGA12通过抑制小胶质细胞和星形胶质细胞的激活来减轻神经炎症。此外,y迷宫测试表明PFGA12有效提高了空间学习记忆能力。从机制上讲,PFGA12通过特异性靶向过氧化物还原素-1 (PRDX1)发挥有效的神经保护作用。PFGA12直接与PRDX1结合,有效抑制Tyr194 (p-PRDX1)的磷酸化,从而增强其过氧化物酶活性。通过谷胱甘肽过氧化物酶4 (GPX4)的上调和酰基辅酶a合成酶长链家族成员4 (ACSL4)的抑制,prdx1介导的抗氧化机制显著减少了脂质活性氧(ROS)的积累,并对OGD/ r诱导的神经元铁凋亡提供保护。值得注意的是,PRDX1的过表达减轻了OGD/R诱导的铁凋亡损伤,而PRDX1的敲低完全消除了PFGA12在OGD/R处理的HT22细胞中的保护作用,证实了PRDX1是PFGA12抑制铁凋亡的关键分子靶点。这些结果表明,PFGA12是一种活性肽,具有作为HIBD新治疗选择的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
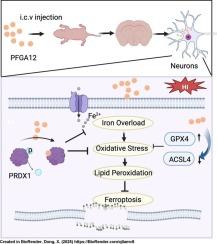
PFGA12 ameliorates Hypoxic-Ischemic brain injury by directly regulating PRDX1 and inhibiting ferroptosis
Hypoxic-ischemic brain damage (HIBD) is a severe condition leading to extensive neuronal loss and functional impairments, representing a significant challenge in neonatal care. PFGA12, a peptide derived from fibrinogen alpha chain (FGA), which is notably downregulated in the umbilical cord blood of hypoxic-ischemic encephalopathy (HIE) infants. We demonstrate that PFGA12 significantly enhances cell viability and mitigates oxygen-glucose deprivation/reperfusion (OGD/R)-induced neuronal cell death. PFGA12 treatment significantly alleviated cerebral edema, reduced infarct volume, and attenuated neuronal damage in HIBD rats, attributable to its stable presence within neurons. Additionally, PFGA12 attenuated neuroinflammation by inhibiting the activation of microglia and astrocytes. Moreover, Y-maze test demonstrated that PFGA12 effectively improved the spatial learning and memory abilities. Mechanistically, PFGA12 exerts potent neuroprotective effects by specifically targeting peroxiredoxin-1 (PRDX1). PFGA12 directly binds to PRDX1, effectively inhibiting phosphorylation at Tyr194 (p-PRDX1), thereby enhancing its peroxidase activity. This PRDX1-mediated antioxidant mechanism substantially reduces lipid reactive oxygen species (ROS) accumulation and provides protection against OGD/R-induced neuronal ferroptosis, as demonstrated by the upregulation of Glutathione peroxidase 4 (GPX4) and suppression of Acyl-CoA synthetase long-chain family member 4 (ACSL4). Notably, overexpression of PRDX1 mitigates ferroptotic damage induced by OGD/R, while knockdown of PRDX1 completely abolishes the protective effects of PFGA12 in OGD/R-treated HT22 cells, confirming PRDX1 as the critical molecular target through which PFGA12 inhibits ferroptosis. These results demonstrate that PFGA12, an active peptide, exhibits potential as a novel treatment option for HIBD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


