亚细亚酸通过靶向ACSS2减少乙酰辅酶a的产生,抑制糖尿病肾病的内皮到间质转化
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
内皮-间充质转化(EndMT)是糖尿病肾病(DKD)中肾纤维化的关键因素。亚洲酸(AA)是一种天然的三萜化合物,具有显著的内皮保护和抗纤维化特性;然而,其对DKD中EndMT的影响尚不清楚。本研究旨在探讨AA对DKD中EndMT的治疗作用及其机制。在体内,AA有效抑制DKD小鼠肾小球EndMT,恢复内皮标志物(CD31和VE-cadherin)的表达,降低间质标志物(α-SMA和Vimentin)的表达。同时,AA可显著降低DKD小鼠肾脏乙酰辅酶a水平,该水平升高与EndMT进展密切相关。机制上,acyl-CoA合成酶短链家族成员2 (ACSS2)被认为是促进乙酰辅酶a生成和组蛋白乙酰化的关键酶,从而促进EndMT。在体外,外源乙酸补充和siRNA介导的ACSS2敲低证实了ACSS2在调控EndMT中的作用。药理抑制ACSS2进一步抑制了EndMT的进展。值得注意的是,分子对接和细胞热移实验显示AA直接与ACSS2结合。AA处理降低了乙酰辅酶a的产生,降低了H3K27乙酰化,恢复了内皮特征,抑制了原发性肾小球内皮细胞和内皮细胞系的间质特征。总的来说,这些发现表明AA通过靶向ACSS2减少乙酰辅酶a的产生来抑制DKD中的EndMT。本研究阐明了AA减轻DKD患者肾纤维化的新机制,并强调了ACSS2作为干预的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
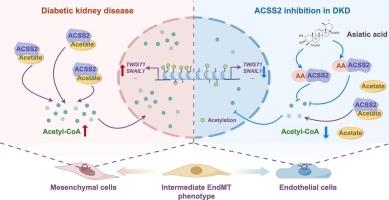
Asiatic acid inhibits endothelial-to-mesenchymal transition in diabetic kidney disease by reducing acetyl-CoA production via targeting ACSS2
Endothelial-to-mesenchymal transition (EndMT) is a critical contributor of renal fibrosis in diabetic kidney disease (DKD). Asiatic acid (AA), a natural triterpenoid compound, exhibits notable endothelial protective and anti-fibrotic properties; however, its impact on EndMT in DKD remains unclear. This study aimed to investigate the therapeutic effect of AA against EndMT in DKD and the underlying mechanisms. In vivo, AA effectively inhibited EndMT in the glomeruli of DKD mice, restored the expression of endothelial markers (CD31 and VE-cadherin), while reduced the expression of mesenchymal markers (α-SMA and Vimentin). Meanwhile, AA significantly reduced renal acetyl-CoA levels, which were elevated in DKD mice and strongly associated with EndMT progression. Mechanistically, acyl-CoA synthetase short-chain family member 2 (ACSS2) was identified as a key enzyme promoting acetyl-CoA production and histone acetylation, thereby facilitating EndMT. In vitro, exogenous acetate supplementation and siRNA mediated-ACSS2 knockdown confirmed ACSS2′s role in regulating EndMT. Pharmacological inhibition of ACSS2 further suppressed the progression of EndMT. Notably, molecular docking and cellular thermal shift assays revealed that AA directly binds to ACSS2. AA treatment reduced acetyl-CoA production, decreased H3K27 acetylation, restored endothelial characteristics, and suppressed mesenchymal features in both primary glomerular endothelial cells and endothelial cell lines. Overall, these findings demonstrate that AA inhibits EndMT in DKD by reducing acetyl-CoA production via targeting ACSS2. This study elucidates a novel mechanism by which AA attenuates renal fibrosis in DKD and highlights ACSS2 as a potential therapeutic target for intervention.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


