靶向KRAS-p38-ATF4轴可通过应激反应调节增强PDBAG1肽对卵巢癌的治疗敏感性。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
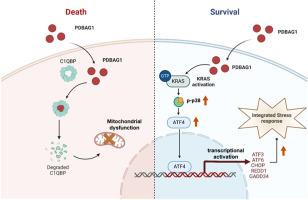
我们之前筛选了一种能显著抑制三阴性乳腺癌的肽PDBAG1,发现其靶点是C1QBP。近年来,C1QBP被报道为卵巢癌的潜在肿瘤标志物,卵巢癌在女性生殖道恶性肿瘤中死亡率居首位。然而,PDBAG1是否以及如何在卵巢癌中发挥调节作用尚不清楚。本研究首次发现PDBAG1在体外和体内均能明显抑制卵巢癌的生长和转移。PDBAG1在卵巢癌中下调C1QBP蛋白水平并破坏线粒体。此外,我们通过转录组学分析了PDBAG1对卵巢癌细胞的整体影响,发现KRAS、炎症和应激相关信号被显著激活。转录组测序结果的准确性随后也得到了验证。此外,我们将KRAS经典下游MAPK信号通路抑制剂和整合应激抑制剂与PDBAG1联合使用,发现p38 MAPK抑制剂Adezmapimod显著增强PDBAG1对卵巢癌的抑制作用,抑制PDBAG1引起的关键应激反应转录因子ATF4的上调。总的来说,我们的研究结果揭示了PDBAG1肽在卵巢癌中的作用和机制,为卵巢癌临床药物开发提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeting the KRAS-p38-ATF4 axis enhances the therapeutic sensitivity of the peptide PDBAG1 in ovarian cancer via stress response modulation
We previously screened a peptide PDBAG1 that remarkably inhibited triple-negative breast cancer, and found that its target was C1QBP. Recently, C1QBP has been reported as a potential tumor marker in ovarian cancer, which of the mortality rate ranks first among malignant tumors of the female reproductive tract. However, it is unclear whether and how PDBAG1 plays a regulatory role in ovarian cancer. Here, we first found that PDBAG1 definitely inhibited the growth and metastasis of ovarian cancer in vitro and in vivo. PDBAG1 downregulated the protein level of C1QBP and damaged mitochondria in ovarian cancer. Furthermore, we analyzed the overall impact of PDBAG1 on ovarian cancer cells through transcriptomics, and found that KRAS, inflammation and stress-related signals were dramatically activated. The accuracy of the transcriptome sequencing results was also subsequently verified. Moreover, we combined the inhibitors of the classic downstream MAPK signaling pathway of KRAS and the integrated stress inhibitor with PDBAG1, and found that the p38 MAPK inhibitor, Adezmapimod, significantly enhanced the inhibitory effect of PDBAG1 on ovarian cancer and inhibited the upregulation of the crucial stress response transcription factor ATF4 caused by PDBAG1. Collectively, our research results revealed the function and mechanism of the peptide PDBAG1 in ovarian cancer, providing new insights into clinical drug development for ovarian cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


