Cr3+掺杂TiO2(金红石)单晶的建模
IF 1.4
4区 化学
Q4 PHYSICS, ATOMIC, MOLECULAR & CHEMICAL
引用次数: 0
摘要
采用叠加模型计算了Cr3+掺杂氧化钛、TiO2(金红石)单晶的晶体场和零场分裂参数。选取了具有畸变的TiO2中Cr3+离子的合适位置进行计算。理论上存在局部畸变的零场分裂参数与实验值吻合较好。利用晶体场分析程序和晶体场参数估计了TiO2中Cr3+的光能带。结果表明,在TiO2单晶中,Cr3+离子取代了其中一个Ti4+离子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
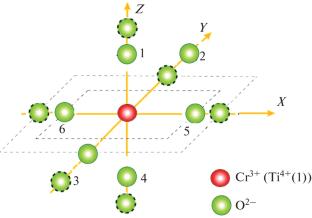
Modeling of Cr3+ Doped TiO2 (Rutile) Single Crystals
Crystal field and zero field splitting parameters of Cr3+ doped titanium oxide, TiO2 (Rutile) single crystals are calculated employing superposition model. The appropriate sites for Cr3+ ions in TiO2 with distortion are taken up for calculation. Splitting parameters of zero fields in theory with local distortion match comparatively well with the values found from the experiment. The optical energy bands for Cr3+ in TiO2 are estimated with the Crystal Field Analysis Program and parameters of the crystal field. The results suggest that Cr3+ ions substitute for one of the Ti4+ ions in TiO2 single crystals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Russian Journal of Physical Chemistry B
化学-物理:原子、分子和化学物理
CiteScore
2.20
自引率
71.40%
发文量
106
审稿时长
4-8 weeks
期刊介绍:
Russian Journal of Physical Chemistry B: Focus on Physics is a journal that publishes studies in the following areas: elementary physical and chemical processes; structure of chemical compounds, reactivity, effect of external field and environment on chemical transformations; molecular dynamics and molecular organization; dynamics and kinetics of photoand radiation-induced processes; mechanism of chemical reactions in gas and condensed phases and at interfaces; chain and thermal processes of ignition, combustion and detonation in gases, two-phase and condensed systems; shock waves; new physical methods of examining chemical reactions; and biological processes in chemical physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


