三种合成化合物的抗利什曼原虫、细胞毒活性和膜刚性效应
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
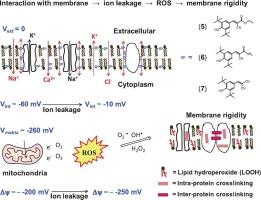
以以查尔酮为核心的先导结构LQFM064和LQFM332为基础,合理设计了三种含有丁基羟基甲苯(BHT)和丙烯酸酯基迈克尔受体支架的抗利什曼类化合物。并对其抗亚马孙利什曼原虫活性进行了评价。丙烯酸酯衍生物(5)、(6)和(7)的IC50值与米替福辛相当,但对J774的细胞毒性明显较低。A1和分化的THP-1巨噬细胞,同时溶血电位降低。自旋标记电子顺磁共振(EPR)光谱显示,这些化合物在24 h后以浓度依赖的方式诱导膜刚性。这种影响不太可能是由于直接的膜相互作用,因为它不会在短时间孵育或低浓度下发生,这表明它与氧化应激有关,如脂质过氧化和/或蛋白质氧化,可能由活性氧(ROS)产生升高引发。相反,在未感染的巨噬细胞中未检测到氧化应激诱导的膜刚性,这表明一氧化氮的产生可能减轻这些细胞的氧化损伤。然而,在利什曼感染的巨噬细胞中,当浓度略高于无尾线虫的IC50时,观察到明显的膜刚性,表明该化合物可能选择性地靶向感染的巨噬细胞。此外,化合物(5)在快速增殖的J774中表现出中等的细胞毒性。但在分化的非增殖性THP-1巨噬细胞中表现出非常低的细胞毒性。总的来说,这项研究表明,这些化合物抗利什曼原虫活性的主要机制与它们对寄生虫质膜的影响有关,可能导致离子泄漏,随后破坏线粒体膜电位,并增强ROS的产生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Antileishmanial, cytotoxic activities, and membrane rigidity effects of three synthetic compounds
Three antileishmanial compounds incorporating a butylated hydroxytoluene (BHT) moiety and an acrylate-based Michael acceptor scaffold were rationally designed from the lead structures LQFM064 and LQFM332, which feature a chalcone-derived core. Their activities against Leishmania (L.) amazonensis were evaluated. Acrylate derivatives (5), (6), and (7) displayed IC50 values comparable to miltefosine, while showing markedly lower cytotoxicity toward J774.A1 and differentiated THP-1 macrophages, along with reduced hemolytic potential. Spin-label electron paramagnetic resonance (EPR) spectroscopy revealed that treatment with these compounds induces membrane rigidity after 24 h in a concentration-dependent manner. This effect is unlikely due to direct membrane interaction, as it does not occur after short incubations or at low concentrations, suggesting a correlation with oxidative stress, such as lipid peroxidation and/or protein oxidation, likely triggered by elevated reactive oxygen species (ROS) production. In contrast, no oxidative stress-induced membrane rigidity was detected in uninfected macrophages, suggesting that nitric oxide production may mitigate oxidative damage in these cells. However, significant membrane rigidity was observed in Leishmania-infected macrophages at concentrations slightly above the IC50 for amastigotes, indicating that the compounds may selectively target infected macrophages. Additionally, compound (5) exhibited moderate cytotoxicity in the rapidly proliferating J774.A1 macrophage line but displayed very low cytotoxicity in differentiated, non-proliferative THP-1 macrophages. Overall, this study suggests that the primary mechanisms underlying the antileishmanial activity of these compounds are associated with their effects on the parasite plasma membrane, potentially leading to ionic leakage, subsequent disruption of mitochondrial membrane potential, and enhanced ROS generation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


