碳离子联合光子放疗通过nco_4介导的铁蛋白吞噬诱导胶质母细胞瘤铁凋亡
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
胶质母细胞瘤(GBM)是中枢神经系统最常见和最致命的原发性恶性肿瘤,由于剂量分布的固有限制,传统的光子放疗仍然难以治疗。尽管碳离子放疗具有明显的优势,包括其特有的布拉格峰沉积和优越的相对生物学有效性,但其临床应用受到成本高和毒性增加的限制。本研究探讨了混合碳离子-光子照射方案背后的放射生物学相互作用,这是一种有前途的先进粒子治疗策略。我们的研究结果表明,联合照射在GBM模型中具有协同细胞毒作用。机制分析表明,这种结合诱导了聚集的DNA双链断裂,导致细胞质中双链DNA (dsDNA)片段的积累。这进而激活cgas - sting介导的胞质DNA传感通路,促进NCOA4-FTH1轴驱动的铁蛋白自噬,最终触发铁依赖性铁凋亡。这些发现为优化GBM联合粒子治疗方案提供了新的机制视角,对临床放射肿瘤学的转化应用具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
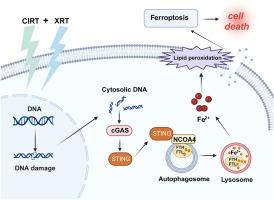
Carbon ion combined photon radiotherapy induces ferroptosis via NCOA4-mediated ferritinophagy in glioblastoma
Glioblastoma (GBM), the most prevalent and lethal primary malignancy of the central nervous system, remains refractory to conventional photon radiotherapy due to inherent limitations in dose distribution. Although carbon ion radiotherapy offers distinct advantages, including its characteristic Bragg peak deposition and superior relative biological effectiveness, its clinical application is constrained by high costs and increased toxicity. This study explores the radiobiological interactions underlying a mixed carbon ion-photon irradiation regimen, a promising strategy in advanced particle therapy. Our findings demonstrate that combined irradiation exerts synergistic cytotoxic effects in GBM models. Mechanistic analysis reveals that this combination induces clustered DNA double-strand breaks, leading to the cytoplasmic accumulation of double-stranded DNA (dsDNA) fragments. This, in turn, activates the cGAS-STING-mediated cytosolic DNA sensing pathway, which facilitates NCOA4-FTH1 axis-driven ferritinophagy and ultimately triggering iron-dependent ferroptosis. These findings offer a new mechanistic perspective on optimizing combined particle therapy regimens for GBM treatment, with significant implications for translational applications in clinical radiation oncology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


