通过直接N-H活化光氧化还原催化合成吲哚以产生假定的氨基自由基
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以可见光激发3,6-二(三氟甲基)-9,10-菲蒽醌(PQ-CF3*)为光催化剂,通过2-乙烯基胺环化合成3-取代n -吡啶吲哚,并与氯(吡啶)钴胺配合物催化再生。该反应的通用性表明,22底物提供高达83%的产率。基于机理研究,我们提出PQ-CF3直接激活N-H键,生成一个以氮为中心的氨基自由基作为关键中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
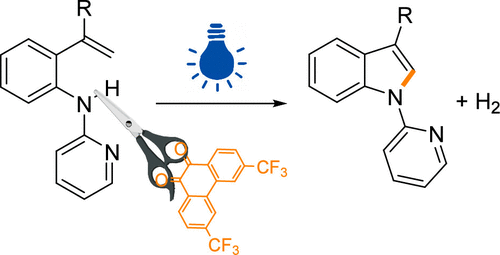
Photoredox Catalytic Synthesis of Indoles via Direct N–H Activation to Generate Putative Aminyl Radicals
Visible-light-excited 3,6-bis(trifluoromethyl)-9,10-phenanthrenequinone (PQ-CF3*) was used as a photocatalyst in the synthesis of 3-substituted N-pyridyl indoles via cyclization of 2-vinylarylamines, where the photocatalyst was catalytically regenerated with the chloro(pyridine)cobaloxime complex. The versatility of the reaction was shown with 22 substrates providing up to 83% yields. Based on the mechanistic studies, we propose that PQ-CF3 directly activates the N–H bond, generating a nitrogen-centered aminyl radical as the key intermediate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


