β-羟基丁酸代谢的另一种途径支持癌细胞胞质乙酰辅酶a的合成
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
癌细胞暴露于肿瘤微环境中的多种代谢物中,这些代谢物用于支持快速细胞增殖所需的核苷酸、氨基酸和脂质的合成。在一些肿瘤中,酮体如β-羟基丁酸(β-OHB),在禁食或低血糖饮食条件下在循环中升高,可以作为替代燃料,在线粒体中代谢,为三羧酸(TCA)循环提供乙酰辅酶a。在这里,我们确定了β-OHB代谢的非规范途径,绕过TCA循环产生细胞质乙酰辅酶a。我们发现,在能够代谢酮的癌细胞中,线粒体中β- ohb衍生的乙酰乙酸可以被分流到细胞质中,在那里乙酰乙酰辅酶a合成酶(AACS)和硫酶将其转化为细胞质乙酰辅酶a。这种替代代谢途径允许β-OHB避免在线粒体中氧化,并作为细胞质乙酰辅酶a的主要来源,即使当其他关键的细胞质乙酰辅酶a前体如葡萄糖过量可用时也是如此。最后,我们证明酮体代谢,包括这种替代的aacs依赖性途径,可以支持小鼠KrasG12D的生长;Trp53 - / -胰腺肿瘤在雄性小鼠的胰腺中原位生长,以及B16黑色素瘤肿瘤在喂食卡路里限制饮食的雄性小鼠中生长。总之,这些数据揭示了癌细胞如何利用β-OHB作为细胞质乙酰辅酶a的主要来源来支持细胞增殖和肿瘤生长。本文章由计算机程序翻译,如有差异,请以英文原文为准。

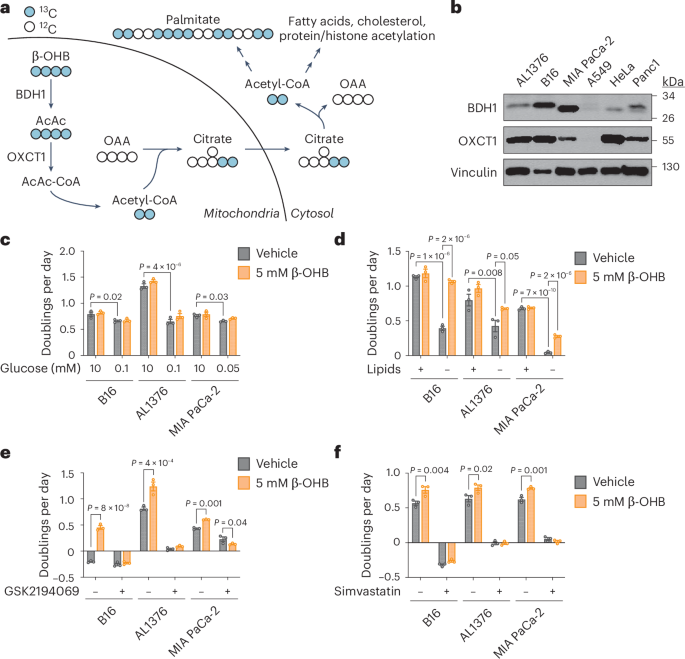
An alternative route for β-hydroxybutyrate metabolism supports cytosolic acetyl-CoA synthesis in cancer cells
Cancer cells are exposed to diverse metabolites in the tumour microenvironment that are used to support the synthesis of nucleotides, amino acids and lipids needed for rapid cell proliferation. In some tumours, ketone bodies such as β-hydroxybutyrate (β-OHB), which are elevated in circulation under fasting conditions or low glycemic diets, can serve as an alternative fuel that is metabolized in the mitochondria to provide acetyl-CoA for the tricarboxylic acid (TCA) cycle. Here we identify a non-canonical route for β-OHB metabolism that bypasses the TCA cycle to generate cytosolic acetyl-CoA. We show that in cancer cells that can metabolize ketones, β-OHB-derived acetoacetate in the mitochondria can be shunted into the cytosol, where acetoacetyl-CoA synthetase (AACS) and thiolase convert it into cytosolic acetyl-CoA. This alternative metabolic routing allows β-OHB to avoid oxidation in the mitochondria and to be used as a major source of cytosolic acetyl-CoA, even when other key cytosolic acetyl-CoA precursors such as glucose are available in excess. Finally, we demonstrate that ketone body metabolism, including this alternative AACS-dependent route, can support the growth of mouse KrasG12D; Trp53−/− pancreatic tumours grown orthotopically in the pancreas of male mice, as well as the growth of mouse B16 melanoma tumours in male mice fed a calorie-restricted diet. Together, these data reveal how cancer cells use β-OHB as a major source of cytosolic acetyl-CoA to support cell proliferation and tumour growth. Kaluba, Rogers et al. show that in cancer cells that can metabolize ketones, they may reroute them to produce cytosolic acetyl-CoA and support tumour growth.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


