在h3k27m改变的DIPG的3D疾病模型中,多细胞肿瘤-基质相互作用概括了治疗反应和人类致癌信号传导的各个方面。
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
从几十年的临床试验中可以明显看出,需要多模式治疗方法,重点关注细胞内在和微环境线索,以提高对罕见的,不可手术的,最终致命的弥漫性脑桥内胶质瘤(DIPG)的理解和治疗,现在被归类为弥漫性中线胶质瘤。在这项研究中,我们报告了利用3D肿瘤组织类似物(TTA)的体外系统的开发和表征,旨在复制复杂的DIPG微环境。荧光标记的人脑内皮细胞、小胶质细胞和患者来源的DIPG细胞系具有自组装的先天能力,已被利用来产生模拟组织样微结构的多细胞3D TTAs,从而能够深入探索肿瘤细胞和基质细胞之间的时空动态。3D-TTA模型概括了DIPG生长的临床模式,证明了对化疗、HDAC和蛋白酶体抑制剂的耐药性,以及对抗体激活的先天免疫微环境(包括补体蛋白和周围的小胶质细胞)的致敏。结合高通量组学的多模态荧光成像平台显示,与肿瘤细胞球体相比,3D-TTA模型中肿瘤细胞运动和生长的改变与特异性转录组学和蛋白质组学变化相关。STAT3、ITGA5、LGALS1、SOD2、MVP和CLIC1与微环境信号、DNA复制和免疫调节相关,在3D模型中被确定为潜在的新靶点。结果表明,本文开发的3D TTA平台是临床前研究的有力工具,为组织特异性生物标志物和新药物靶点的鉴定/验证铺平了道路,从而推进了儿童DIPG的疾病管理策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
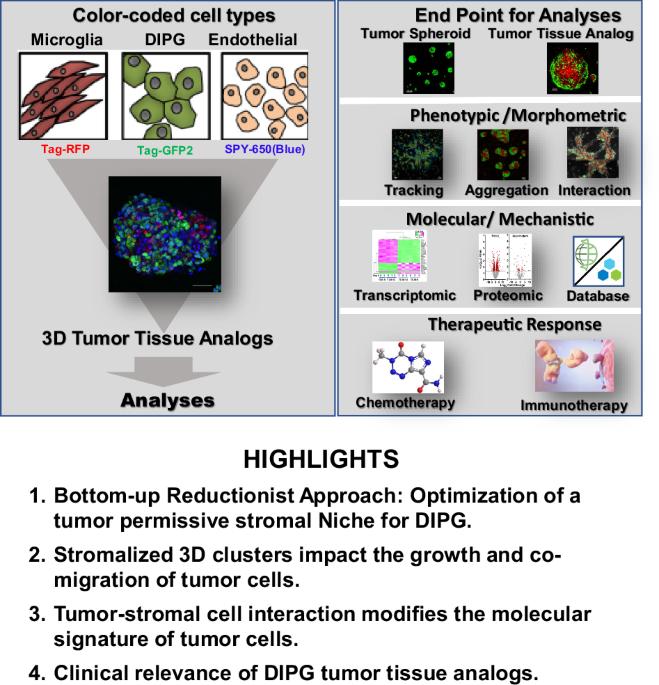
Multicellular tumor-stromal interactions recapitulate aspects of therapeutic response and human oncogenic signaling in a 3D disease model for H3K27M-altered DIPG
It has become evident from decades of clinical trials that multimodal therapeutic approaches with focus on cell intrinsic and microenvironmental cues are needed to improve understanding and treat the rare, inoperable, and ultimately fatal diffuse intrinsic pontine glioma (DIPG), now categorized as a diffuse midline glioma. In this study we report the development and characterization of an in vitro system utilizing 3D Tumor Tissue Analogs (TTA), designed to replicate the intricate DIPG microenvironment. The innate ability of fluorescently labeled human brain endothelial cells, microglia, and patient-derived DIPG cell lines to self-assemble has been exploited to generate multicellular 3D TTAs that mimic tissue-like microstructures, enabling an in- depth exploration of the spatio-temporal dynamics between neoplastic and stromal cells. The 3D-TTA model recapitulates clinical patterns of DIPG growth, evidenced by resistance to chemotherapy, HDAC and proteasome inhibitors, as well as sensitization to the antibody-activated innate immune microenvironment including complement proteins and surrounding microglia. Multimodal fluorescence imaging platforms integrated with high-throughput omics revealed that alterations in tumor cell motility and growth in the 3D-TTA model compared to tumor cell only spheroids correlated with specific transcriptomic and proteomic changes. STAT3, ITGA5, LGALS1, SOD2, MVP, and CLIC1, associated with microenvironment signaling, DNA replication, and immune regulation, were identified as potential novel targets in the 3D model. The results indicate that the 3D TTA platform developed here represents a powerful tool for preclinical studies, paving the way for identification/validation of tissue specific biomarkers and novel drug targets, thus advancing disease management strategies for DIPG in children.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


