纳米药物抑制转移和诱导细胞凋亡以减轻治疗耐药癌症的复发。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
癌症是全球死亡的主要原因之一,主要受到化疗和放疗等传统疗法的耐药性和毒性的影响。最近的研究表明,细胞从接近死亡状态中恢复是促进癌症复发和细胞凋亡抵抗的关键机制。在转移过程中,应激诱导的半胱天冬酶激活使癌细胞存活,增加化疗耐药,并增强转移潜力。热休克蛋白(HSPs)通过修复受损蛋白质和维持细胞在压力下的活力来增强这种恢复能力。本综述提出了基于纳米技术的新策略,通过使用先进的纳米颗粒将热休克蛋白抑制剂和半胱氨酸酶调节剂直接靶向肿瘤,从而破坏这些生存途径。通过关注转移和凋亡之间的相互作用,我们的方法旨在抑制使癌细胞逃避死亡的机制,同时提高治疗的精确性和最小化全身毒性。这些纳米技术增强的策略有望克服治疗耐药性,并带来更安全、更有效的抗癌疗法。这样的创新可以大大提高我们对癌症细胞死亡和存活的理解,为下一代治疗干预铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
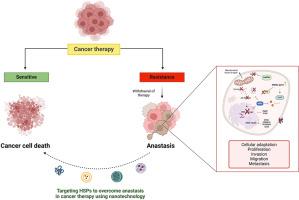
Nanomedicine solutions for inhibiting anastasis and inducing apoptosis to mitigate relapse in treatment-resistant cancers
Cancer is a leading cause of global mortality, significantly impacted by treatment resistance and the toxicity of conventional therapies like chemotherapy and radiation. Recent studies show that anastasis—the recovery of cells from near-death states—as a key mechanism promoting cancer relapse and apoptosis resistance. During anastasis, stress-induced caspase activation allows cancer cells to survive, increase chemoresistance, and enhance metastatic potential. Heat shock proteins (HSPs) reinforce this resilience by repairing damaged proteins and maintaining cell viability under stress. This review presents novel nanotechnology-based strategies that disrupt these survival pathways by targeting HSP inhibitors and caspase modulators directly to tumors using advanced nanoparticles. By focusing on the interplay between anastasis and apoptosis, our approach aims to inhibit the mechanisms that enable cancer cells to evade death while enhancing treatment delivery precision and minimizing systemic toxicity. These nanotech-enhanced strategies promise to overcome treatment resistance and lead to safer, more effective anticancer therapies. Such innovations could significantly advance our understanding of cell death and survival in cancer, paving the way for next-generation therapeutic interventions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


