利用不同的光谱平台绘制LCD-TDP43在液-液相分离过程中的结构变化
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
全面了解LCD-TDP43液-液相分离(LLPS)途径的分子机制仍然是其神经发病机制的一个挑战。TDP-43 LLPS背后的主要驱动力是芳香残基增强的疏水相互作用的相互作用。本研究提出了一种新颖、方便、灵敏、无探针的方法,利用激发-发射矩阵(EEM)荧光来监测LLPS途径不同阶段芳香族残基和π-π堆积相互作用的微环境。蛋白质局部结构和芳香残基位置的变化,单独和集体,通过这种终身3D指纹检测。通过CD和FTIR分析,发现了液滴态中具有独特α-片结构的新中间态和淀粉样原纤维等瞬态物质。这种结构具有转化为β-淀粉样蛋白的固有倾向,以前在LCD-TDP43或其他llps易感蛋白的背景下尚未报道。在相分离过程中疏水聚类的映射显示出连续的增加,并伴随着不同的周围极性。AFM分析和ThT试验也证实了LLPS途径(从单体到纤维)中不同蛋白质种类的形成,以及TDP-43纤维的淀粉样变性。总之,本研究中引入的3D荧光方法提供了一种有效而直接的方法,可以深入了解含有lcd的IDPs的llps依赖性聚集途径中关键的π-π相互作用。α-片非原纤维中间体的新鉴定可能为阐明这些蛋白的聚集机制提供新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
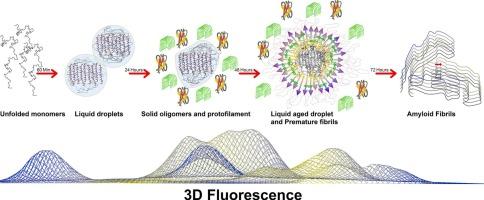
Mapping the structural changes of LCD-TDP43 during the liquid-liquid phase separation by different spectroscopic platforms
A comprehensive understanding of the molecular mechanism underlying the Liquid-Liquid Phase Separation (LLPS) pathway of LCD-TDP43 remains a challenge in the context of its neuropathogenesis. The primary driving force behind the TDP-43 LLPS is the interplay of hydrophobic interactions reinforced by aromatic residues. This study presents a novel, convenient, sensitive, and probe-free approach using excitation-emission matrix (EEM) fluorescence to monitor the microenvironment of aromatic residues and π-π stacking interactions during different stages of the LLPS pathway. Protein local structuring and the alterations in the positions of aromatic residues, individually and collectively, were detected by this life-time 3D fingerprinting. A new intermediate state with a unique α-sheet structure in the liquid droplet state and other transient species up to amyloid fibrils was discovered by CD and FTIR analyses. This structure with an inherent tendency for transition to β-amyloids, has not previously been reported in the context of LCD-TDP43 nor other LLPS-prone proteins. Mapping of hydrophobic clustering during phase separation revealed a continuous increase, accompanied by different surrounding polarities. The formation of distinct protein species within the LLPS pathway (from monomer to fibril), along with the amyloidogenic nature of TDP-43 fibrillation, was also confirmed by AFM analysis and ThT assay. To conclude, the 3D fluorescence method introduced in this study provides an effective and straightforward approach to critical valuable insights into the key π-π interactions in the LLPS-dependent aggregation pathway of LCD-containing IDPs. The novel identification of the α-sheet non-fibrilar intermediates may provide a new perspective for elucidating the aggregation mechanism of these proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


