Pde5a缺乏通过激活脂肪cAMP-PKA促进脂肪褐变来预防饮食引起的肥胖。
IF 6.6
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
环核苷酸是适应性产热和脂肪生成的关键调节因子,其细胞内水平由磷酸二酯酶精细调节。磷酸二酯酶5 (PDE5A)调节脂肪细胞中的环鸟苷单磷酸(cGMP)水平。虽然PDE5A抑制已在糖尿病患者中显示出前景,但其在代谢中的作用仍不清楚。使用不同的Pde5a敲除小鼠模型,我们证明Pde5a的缺失导致棕色脂肪组织的强烈激活和白色脂肪组织的中度褐化,同时伴随着肝脏脂肪含量的降低。在高脂肪饮食后,Pde5a缺陷小鼠对肥胖有抵抗力,表现出改善的糖代谢和增强的产热能力。这些保护作用源于Pde5a的早期发育敲低,导致camp蛋白激酶a (PKA)途径激活驱动的代谢重编程。cGMP和cAMP信号的融合协调了产热和全身代谢适应。我们的研究结果表明PDE5A是一种新的能量稳态调节因子,表明抑制PDE5A是一种有价值的代谢紊乱的辅助治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
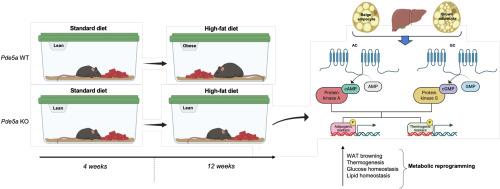
Pde5a deficiency prevents diet-induced obesity via adipose cAMP-PKA activation enhancing fat browning
Objective
Cyclic nucleotides are central regulators of adipogenesis and adaptive thermogenesis, with their intracellular concentrations tightly controlled by phosphodiesterases (PDEs). Among them, phosphodiesterase type 5 (PDE5A) regulates cyclic guanosine monophosphate (cGMP) turnover in adipocytes. Although PDE5A inhibition has been explored in diabetes, its role in systemic metabolism remains poorly defined.
Methods
We employed different Pde5a knockout mouse models to investigate the impact of PDE5A deficiency on adipose tissue biology and whole-body energy homeostasis. Phenotypic, histological, and metabolic assessments were performed under chow and high-fat diet conditions, with a focus on thermogenic activation, hepatic lipid accumulation, and glucose metabolism.
Results
Loss of Pde5a resulted in robust activation of brown adipose tissue and moderate browning of white adipose depots, accompanied by a reduction in hepatic lipid content. Upon high-fat diet challenge, Pde5a-deficient mice exhibited resistance to obesity, improved glucose handling, and enhanced thermogenic capacity. Mechanistically, these protective effects originated from early developmental knockdown of Pde5a, which induced metabolic reprogramming via activation of the cAMP–protein kinase A (PKA) signaling pathway. The convergence of cGMP and cAMP signaling cascades orchestrated systemic metabolic adaptations.
Conclusions
Our study identifies PDE5A as a previously unrecognized regulator of thermogenesis and energy balance. Targeting PDE5A may therefore represent a promising adjuvant therapeutic approach for the treatment of metabolic disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


