STAT1-VAMP8轴通过自噬增强驱动鼻咽癌进展。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
鼻咽癌是一种侵袭转移风险高的恶性肿瘤。阐明鼻咽癌的分子基础可能会发现新的诊断和治疗靶点。囊泡相关膜蛋白8 (VAMP8)在多种肿瘤中过表达并起致瘤作用。然而,其在NPC中的作用和潜在机制尚不清楚。在本研究中,我们发现与NPC患者的邻近组织相比,VAMP8在NPC公共数据库(GSE150430和GSE162025)和NPC组织中显著过表达,并且VAMP8过表达显著加速肿瘤生长。溶酶体相关基因在该肿瘤中起主要的致癌作用。进一步的机制研究表明,VAMP8通过促进自噬参与鼻咽癌的发展。此外,我们证明了STAT1转录上调VAMP8, VAMP8过表达挽救了由STAT1敲低介导的肿瘤进展相关表型。基于NPC的药敏分析表明STAT1对氟达拉滨敏感。氟达拉滨抑制STAT1/VAMP8轴可显著抑制鼻咽癌细胞的增殖和转移。总的来说,我们的研究结果表明STAT1-VAMP8轴是通过自噬激活鼻咽癌增殖和转移的关键驱动因素。氟达拉滨有望成为鼻咽癌患者的潜在治疗药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
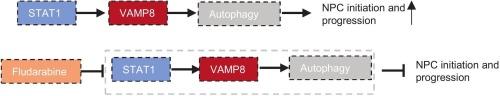
STAT1–VAMP8 axis drives nasopharyngeal carcinoma progression via autophagy enhancement
Nasopharyngeal carcinoma (NPC) is a malignant tumor with a high risk of invasion and metastasis. Elucidating the molecular underpinnings of NPC may uncover new diagnostic and therapeutic targets. Vesicle associated membrane protein 8 (VAMP8) is overexpressed and plays an oncogenic role in various tumors. However, its role and underlying mechanism in NPC remains unclear. In this study, we identified that VAMP8 is significantly overexpressed in NPC public databases (GSE150430 and GSE162025) and NPC tissues compared to adjacent tissues in NPC patients, and VAMP8 overexpression markedly accelerated tumor growth. Lysosome associated genes play major carcinogenic roles in this tumor. Further mechanistic investigations revealed that VAMP8 was involved in the development of NPC by promoting autophagy. Furthermore, we demonstrate that STAT1 transcriptionally up-regulates VAMP8, and VAMP8 overexpression rescued the tumor progression-related phenotypes mediated by STAT1 knocking down. Drug sensitive analysis based on NPC indicated that STAT1 is sensitive to fludarabine. Pharmacologically inhibiting STAT1/VAMP8 axis by fludarabine significantly suppressed the NPC cell proliferation and metastasis. Collectively, our findings establish the STAT1–VAMP8 axis as a critical driver of NPC proliferation and metastasis through autophagy activation. Fludarabine is expected to be a potential therapeutic agent in NPC patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


